Research Article - (2025) Volume 10, Issue 1
Objective: Methamphetamine (Met) is a highly addictive psychostimulant that elevates the amount of dopamine in the brain. Dopamine accumulation leads to oxidative stress and eventually induces brain damage. Alpha-Lipoic Acid (ALA) is a potent natural antioxidant in the body, and reduction of oxidative stress by ALA has been shown in animal models. In this study, we investigated the effects of intraperitoneal administration of alpha-lipoic acid on anxiety-like behaviors, pain perception, and oxidative stress in the periaqueductal gray in Met-induced neurotoxicity.
Methods: Thirty-five male Wistar rats were divided into five equal groups: 1) saline+saline, 2) saline+vehicle (sunflower oil as ALA solvent), 3) Met+vehicle, 4) Met+ALA (10 mg/kg), and 5) Met+ALA (40 mg/kg). Rats received methamphetamine repeatedly (2 × 20 mg/kg, 2 hour interval), and alpha-lipoic acid was injected 30 minutes, 24 hours, and 48 hours after the last methamphetamine injection. We used the tail flick test to evaluate pain perception and the open field test to evaluate anxiety-like behaviors. After the behavioral tests, we anesthetized the rats, extracted their brains, prepared periaqueductal gray matter homogenates, and evaluated Malondialdehyde (MDA) level, Catalase (CAT), and Superoxide Dismutase (SOD) activities.
Results: Met administration not only induced hyperalgesia and anxiety-like behaviors, but also increased MDA levels and reduced CAT and SOD activities in the periaqueductal gray matter area. Treatment with ALA decreased MDA levels, increased CAT and SOD activities, and prevented hyperalgesia and anxiety-like behaviors.
Conclusion: Our results showed that Met administration induced hyperalgesia, anxiety-like behaviors, oxidative stress, and impaired antioxidant defenses in the PAG. Administration of ALA decreased oxidative stress in the periaqueductal gray matter area by elevating SOD and CAT activity and reduced hyperalgesia and anxiety-like behaviors following acute Met administration.
Methamphetamine • Alpha-lipoic acid • Periaqueductal gray • Oxidative stress • Anxiety • Pain
Methamphetamine (Met) is a potent psychostimulant that is abused by 35 million people worldwide [1]. The growing prevalence of this drug has raised serious concerns about increasing numbers of associated health complications and mortality among young adults. High doses of Met induce neurotoxicity, which can cause significant medical consequences, such as Parkinson's disease, seizures, visual and auditory hallucinations, depression, and anxiety [2].
It has been proposed that neuroinflammation, inability to regulate intracellular calcium, alterations in neurotransmission, apoptosis, and oxidative stress are involved in Met neurotoxicity. The major neurotoxic effect of Met on the brain is linked to oxidative stress. Oxidative stress plays a significant role in the pathogenesis of atherosclerosis, cancer, diabetes, Alzheimer's, and Parkinson's diseases [3]. It has been shown that Met easily diffuses through the blood-brain barrier and affects dopaminergic terminals. It increases dopamine concentration in the synapses by massive release of dopamine or by inhibiting vesicular monoamine transporter 2 at the end of axons. Then, autoxidation of dopamine molecules increases Reactive Oxygen Species (ROS) in the brain. ROS initiate the peroxidation of membrane lipids and promote neuronal death by destroying the cell membrane. Furthermore, ROS increase cytochrome C entry into the cytoplasm and initiate apoptosis by activating caspase-3 enzyme or BAX [4].
Brain dopaminergic neurons project into the spinal cord and the existence of dopamine receptors in different laminae of the spinal cord suggests that dopamine can modulate pain signals by acting at both presynaptic and postsynaptic targets. Animal studies have provided exciting evidence that dopamine can modulate the intrinsic excitability and synaptic transmission of spinal neurons involved in pain signaling. Moreover, dopamine is an important neurotransmitter involved in the behavioral response to stress, and its transmission is critical for modulating anxiety behaviors [5].
It is well-known that cells have an antioxidant defense system, including vitamin E, glutathione, Superoxide Dismutase (SOD), and Catalase (CAT). It has been revealed that administration of Met decreases SOD and CAT activity in neurons. Because antioxidants neutralize free radicals, they can prevent the aversive effects of oxidative stress. Alpha-Lipoic Acid (ALA), as a cofactor, is present in the mitochondrial multi-enzyme complex, which catalyzes decarboxylation of α-ketoglutarate and pyruvate. Because ALA is soluble in both water and fat, it quickly absorbs from the gastrointestinal tract and enters the brain. It has been reported that ALA protects neurons against cerebral ischemia, reduces inflammation and promotes neuronal regeneration. ALA is a potent antioxidant that protects neurons against oxidative stress and reverses memory impairments in aged animals [6].
Pain sensation depends on the precise control of sensory conduction from the spinal cord to the brain. The Periaqueductal Gray matter (PAG) surrounds the Sylvius aqueduct in the midbrain and plays a main role in pain modulation and anxiety. Catecholamine neurons of the PAG represent an important neuro anatomical substrate for the behavioral changes induced by Met. The PAG receives input from the cortex, amygdala, and hypothalamus and sends its projections to other brain regions, such as the Ventrolateral Medulla (VlM) and Locus Coeruleus (LC). The PAG-LC pathway induces analgesia in the spinal dorsal horn via alpha-2 receptors. Also, the serotonergic PAG-VlM pathway is considered the major modulatory pain pathway and constitutes a primary target for supraspinal opioid analgesia. It has been demonstrated that activation of the amygdala-PAG pathway increases anxiety during the elevated plus maze test. Serotonin, dopamine, enkephalin, and glutamate are found in high concentrations in the PAG. Serotonin deficit in the PAG may contribute to the development of anxiety, and its elevation mediates the anxiolytic action of antidepressant drugs. Studies in the brains of Met abusers have shown that Met abuse targets dopamine neurons in the PAG. Also, repeated administration of Met induces changes in the plasticity of PAG dopaminergic neurons that are associated with drug-induced reward and addiction. Further, it has been reported that injection of Met decreases Dopamine Transporter (DAT), Serotonin Transporter (5-HTT), Monoamine Transporter Type 2 (VMAT-2) and activates glial cells in the PAG [7].
As the effects of Met in the CNS are mainly dependent on its interaction with the dopaminergic system and oxidative stress, it was expected that exposure to Met could modify nociception and induce anxiety. Furthermore, it was assumed that an antioxidant such as ALA may prevent the adverse effects of Met. To test this hypothesis, the effects of intraperitoneal injection of ALA on pain perception and anxiety in Met-induced neurotoxicity and its ability to prevent oxidative stress in the PAG were investigated.
Animals
To evaluate the protective effect of ALA, 40 male Wistar rats (180-200 g) were obtained from Pasteur institute (Tehran, Iran). The animals were maintained at standard laboratory conditions (12 hours of light and dark cycle (light on from 07:00 am to 19:00 pm), temperature 20-22°C and 40% humidity) and provided food and water ad libitum. Animals were allowed to become accustomed to the new environment and were handled for a week prior to tests. In the present study, the behavioral tests were performed during the daytime period (from 9:00 to 12:00 am). All efforts were made to minimize the number of animals used and prevent their suffering. Animals allocated randomly in the 5 groups (n=8 per group): 1) saline +saline, 2) saline+vehicle (sunflower oil as ALA solvent), 3) Met +vehicle, and two Met groups under treatment with ALA (10 and 40 mg/kg). Met was dissolved in sterile saline and injected repeatedly (2 × 20 mg/kg, 2 hour interval) [8]. ALA was injected 30 min, 24 h and 48 h after the last Met injection. ALA doses were chosen based on literature data and our pilot study. ALA dissolved in sunflower oil as vehicle. Normal saline, sunflower oil, Met and ALA were administered intraperitoneally.
Chemicals
Thiobarbituric acid, Malondialdehyde Tetrabutylammonium salt (MDA salt), methanol, glacial acetic acid was purchased from Merck Company (Darmstadt, Germany). All other chemicals were purchased from Sigma-Aldrich (St. Louis, USA).
Behavioral test
Tail flick test: Anti-nociception was assessed by the tail flick test, using an automatic analgesio meter (Tail flick; Borj Sanat, Tehran, Iran). The latency time was measured as the time between tail exposure to radiant heat and tail withdrawal. A cut-off time of 10 sec was used throughout the study. The latency time was derived from the mean of three tests with an interval of 2 min between readings.
Open field test: The open field test is a standard measure of anxiety anxiety-like behavior, taking advantage of a rat’s normal aversion to exploration of a central zone and the preference to spend time around the peripheral zone. The open field apparatus consisted of a 100 × 100 cm square arena with 40 cm high walls which were made of plastic board (Borj Sanat, Tehran, Iran). The main variables recorded during the next 5 minutes were:
• Locomotor activity
• Time spent in the central zone
• Number of entries into the central zone
• Time spent in corners
• Number of entries into the corner
At the beginning of the trial, each rat was placed in the center of the arena and allowed to freely explore. All processes were recorded by a camera (Canon 3100 IS, Japan) and saved on a personal computer. During the test session, the total distance moved (locomotor activity), time spent in the central zone and grooming behavior were measured automatically by ANY-maze software (Stoelting Co., Wood Dale, IL). It has been proposed that in the anxious animal locomotor activity and number of defecation boluses are elevated while central zone time, rearing and grooming are reduced [9].
Assessment of biochemical parameters
Tissue sampling: After behavioral test, rats were anesthetized using ketamine hydrochloride (100 mg/kg) and xylazine (5 mg/kg) combination. The brain was removed quickly from skull and then brain stem under superior and inferior colliculus was cut and PAG was microdissected carefully. PAG was washed with saline, dried on filter paper and finally weighed. Then, 10% (w/v) PAG homogenate was prepared in ice-cold phosphate buffer (50 mM, pH 7.4). The supernatant obtained following the centrifugation of homogenate at 4000 g for 10 minutes and was used for measuring of protein, MDA, CAT and SOD activities. The protein concentration was determined using the Bradford method [10].
Assay of lipid peroxidation: Tissue Malondialdehyde (MDA) level was measured by Ohkawa’s method. This method evaluates the ability of MDA to react with thiobarbituric acid in acidic conditions and producing the pink color. Briefly, 0.3 ml PAG homogenate were added to 0.2 ml sodium dodecyl sulfate (8.1%), 1.5 ml of 20% acetic acid, 1.5 ml TBA (0.8%) and distilled water (0.5 ml). This mixture was heated in water bath at 95°C for 60 min. After cooling with tap water, absorbance at λ=532 nm was measured [11].
Measurement of SOD and CAT activities: The activity of SOD was determined by the method described by Beauchamp and Fridovich. Briefly, PAG homogenate (0.3 ml) was added to 1.3 mM riboflavin (1.5 ml), 13 mM methionine (1.5 ml) and 63 mM NBT (1.5 ml). The mixtures were illuminated in test tubes for 10 minutes. The reaction was began and finished by turning the light on and off respectively. There was no detectable amount of the reaction occurring under room light during the preparation of the solutions. Finally, absorbance at λ=560 nm was measured.
The activity of CAT was determined by the method described by Yi Li and Schell horn [12]. Briefly, 0.3 ml PAG homogenate were added to 30 mM hydrogen peroxide, then immediately scanned in a spectrophoto meter at λ=240 nm every 10 sec for 5 min at 22°C. Catalase activity was calculated based on the rate of decomposition of hydrogen peroxide which is proportional to the reduction of the absorbance at 240 nm.
Statistical analyses
After normality analysis by using Kolmogorov-Smirnov test, in the parametric test, comparison of experimental groups was performed by one way ANOVA followed by LSD post hoc test (SPSS software). Pearson’s coefficient of correlation was used to analyze relationships between latency time and MDA level. The results are shown as mean ± SE.
Ethical statement
Rats received humane care according to the criteria outlined in the “Guide for the care and use of laboratory animals” (National Institute of Health Publication no. 80-23, revised 1996) and helsinki declaration (1964 and its later amendments). Furthermore, instructors of ethics committee controlled the animal caring and experimental procedures.
All efforts were made to minimize the number of animals used and their suffering. All protocols were approved by the ethics committee of Islamic Azad University, (IR.IAU.SHAHROOD.REC.1400.064) [13].
Tail flick test
Statistical analysis showed no significant difference between Sal +Sal and Sal+Veh groups in the latency time. Injection of Met decreased significantly latency time (P<0.04, Figure 1). Alpha-lipoic acid significantly (one way ANOVA analysis, followed by LSD post hoc) increased the latency time at 10 mg/kg (P<0.01) and 40 mg/kg doses (P<0.02).
Open field test
There was no significant change between (Sal+Sal) and (Sal+Veh) groups in the locomotor activity, spending time in central zone, central zone entering, corner zone entering and rearing. Injection of Met increased locomotor activity (P<0.006, corner zone entering (P<0.002) and decreased spending time in central zone (P<0.002), central zone entering (P<0.001) and rearing (P<0.03) significantly compared to the (Sal+Veh) group. Alpha-lipoic acid treatment significantly (one-way ANOVA analysis, followed by LSD post hoc) decreased locomotor activity at 10 mg/kg (P<0.008) and 40 mg/kg doses (P<0.02), corner zone entering at 40 mg/kg dose (P<0.005) and increased the spending time in central zone (P<0.02) at 40 mg/kg dose, central zone entering at 40 mg/kg doses (P<0.02) and rearing at 40 mg/kg dose (P<0.02), significantly [14].
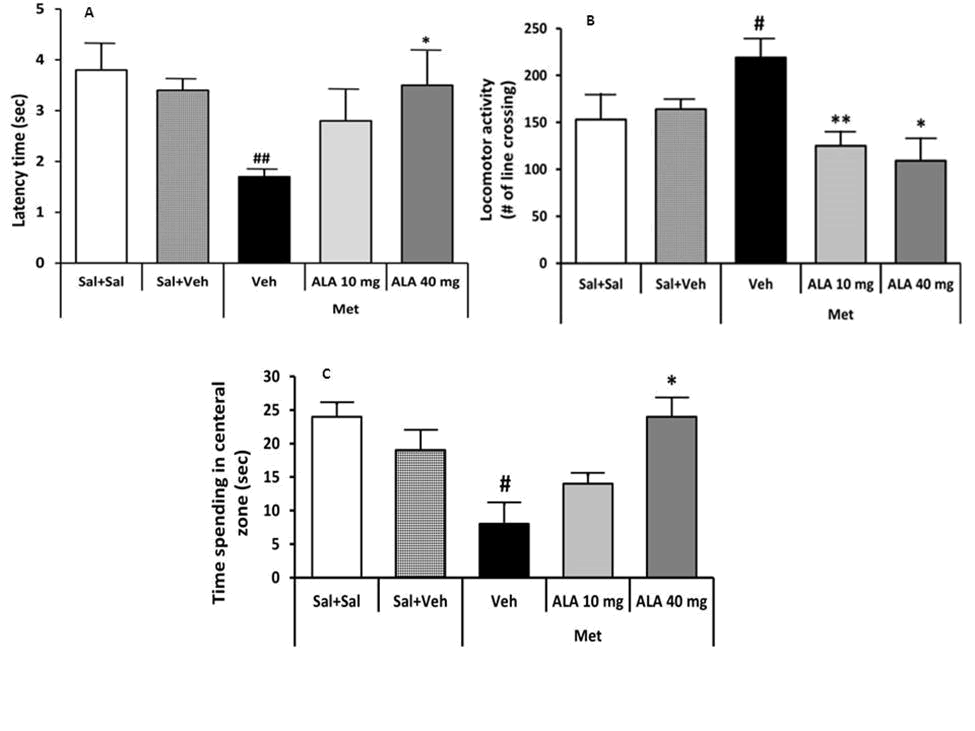
Figure 1. (A): The effect of Methamphetamine (Met) and Alpha Lipoic Acid (ALA) on tail flick latency; (B): Locomotor activity: (C): Spending time in the central zone. Data were presented as mean ± SEM (n=7, #P<0.05, #P<0.01 and ##P<0.001 versus Sal+Veh group; *P<0.05 and **P<0.01 versus Met+Veh group).
Lipid peroxidation
There was no significant difference in MDA level, SOD and catalase activity between (Sal+Sal) and (Sal+Veh) groups (Figure 2A-2C). Therefore, saline or vehicle administration had not affected these paraMeters. MDA level was enhanced significantly by Met compared to the (Sal+Veh) group (P<0.03, Figure 2A). Our analysis showed that ALA treatment significantly reduced MDA level at 40 mg/kg (P<0.015) compared to the (Met+Veh) group (Figure 2A). Furthermore, simple regression analysis (Figure 2D) revealed that hippocampal lipid peroxidation significantly negatively correlated with latency time in the tail flick test (r=0.63, P<0.0003).
SOD and CAT activity in the PAG
There was no significant change in SOD and CAT activities between (Sal+Sal) and (Sal+Veh) groups. Met administration significantly decreased SOD and CAT activities (P<0.001 and P<0.011, Figure 2B and 2C) respectively in the PAG. Further statistical comparison by one-way ANOVA followed by LSD test showed that ALA treatment significantly elevated catalase activity at both 10 mg/ kg (P<0.036) and 40 mg/kg doses (P<0.02, Figure 2C) and also SOD activity only at 40 mg/kg dose (P<0.02, Figure 2B).
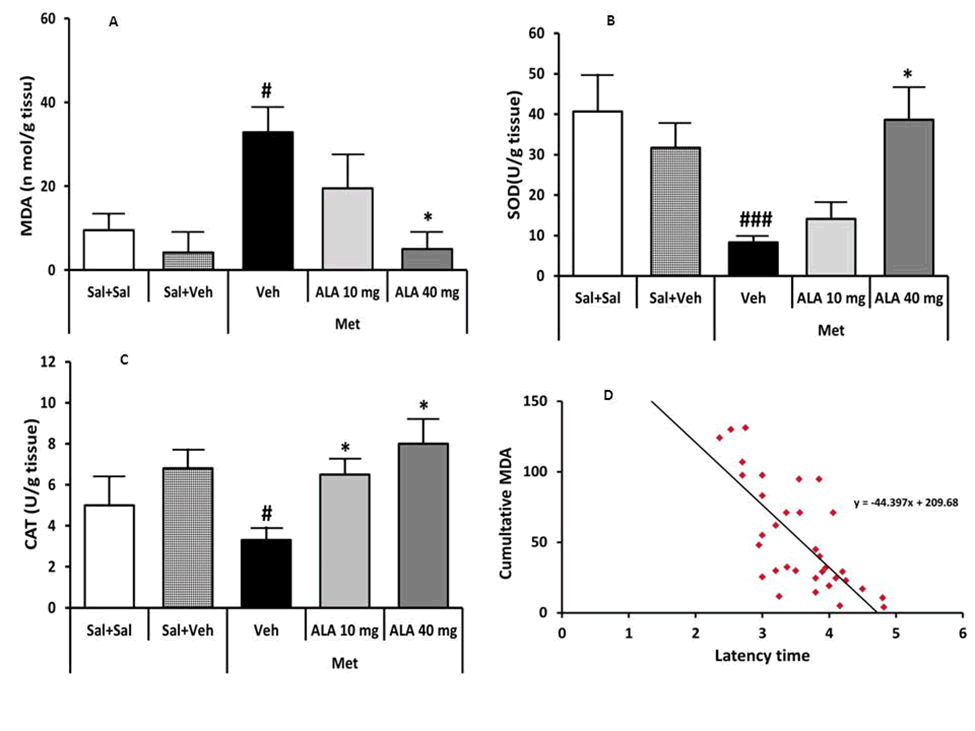
Figure 2. (A): The effect of Methamphetamine (Met) and Alpha Lipoic Acid (ALA) on MDA; (B): SOD activity; (C): CAT activity; (D): Relationship between MDA and latency time. Data were presented as mean ± SEM (n=7, #P<0.05 and ###P<0.001 versus Sal+Veh group; *P<0.05 and versus Met+Veh group).
Met, a strongly addictive psychoactive substance with destructive effects on the nervous system has become more prevalent than other psychostimulants. Its abuse is closely linked to several neuropsychiatric disorders such as anxiety, depression, and memory loss [15]. Our recent study showed that high acute doses of Met increase locomotor activity, entry into corner zone and decrease spending time in the central zone, entry into central zone, rearing and also induce hyperalgesia in rats. It has been proposed that in anxious animals, locomotor activity and number of corner entering are elevated while spending time in the central zone, rearing and grooming are decreased in the open field test. This finding is in line with other reports that have indicated that administration of Met strongly increases anxiety and locomotor activity. However, there are conflicting reports for the effect of methamphetamine on locomotor activity in animals [16]. The effect of Met on locomotor activity may be influenced by various factors, such as species, age, dosage, and the time elapsed between the last dose of Met and the behavioral testing [17].
The neurotoxic effects of Met lead to the destruction of dopaminergic and serotoninergic neurons in various brain areas, including the striatum, nucleus accumbens and nigrostriatal pathway. Consequently, a reduction in dopamine levels in these areas is expected to result in a decrease in motor activity [18]. Met users show high levels of anxiety and it even increases feelings of anxiety in healthy non-drug users. Although, the mechanism of Met-induced nanxiety remains unclear, it is proposed that Hypothalamic-Pituitary Adrenal (HPA) axis may play a major role in the initiation of anxiety. Evidence suggests that Met may increase corticosterone levels or alter serotonin, dopamine and norepinephrine levels which finally lead to anxiety. It has been reported that serotoninergic neurons in the PAG may affect the HPA axis directly. In this regard, it was shown that stimulation of dorsal PAG induces acute signs of autonomic arousal and anxiety.
The result of the tail flick test revealed that acute injections of Met significantly decreased the reaction time to the delivered stimulus. It was reported that prenatal exposure to the Met increases the formalin pain score at the second phase which suggests increased spinal or supraspinal sensitization to pain responses. To our knowledge, this is the first report of hyperalgesia in the acute administration of Met. The PAG is the main center in the CNS that involves in the supraspinal modulation of pain. Animal studies have shown that electrical stimulation of the PAG induces analgesia and blocks ascending pain signals in the spinal cord. This is mediated by PAG-raphe magnus connections in the brainstem. It has been clarified that norepinephrine, dopamine and glutamate contribute in the pain processing in the PAG. Also, recent studies have suggested that the activation of dopaminergic neurons in the Ventral Tegmental Area (VTA) that extend to the Nucleus Accumbens (NAc), plays an essential role in the suppression of pain. To understand the hyperalgesic effects of Met, it is important to take into account that VTA neurons receive nociceptive information and are involved in pain modulation [19]. Our statistical analysis revealed that PAG lipid peroxidation significantly negatively correlated with latency time in the tail flick test. Thus, it is possible that acute high doses of Met, increase the extracellular concentrations of dopamine in the NAc or PAG and destroy end terminals of dopaminergic neurons, leading to hyperalgesia.
Substantial evidence indicates that the administration of Met stimulates dopaminergic, serotoninergic, and specially glutamatergic systems. Furthermore, it has been shown that excessive release of glutamate leads to glutamate-mediated neurotoxicity, which leads to the activation of calcium-dependent proteolytic enzymes and finally increases ROS production. Neuronal damage by free radicals is considered the main mechanism for Met neurotoxicity. According to our biochemical analysis Met administration increased MDA concentration and decreased SOD and catalase activities in the PAG. Consistent with our findings, several reports have shown that Met raises MDA levels in human or animal brains. Moreover, Met causes a huge release of dopamine in the brain by degenerating dopaminergic terminals. Dopamine then reacts with molecular oxygen to form ROS and initiates the peroxidation of membrane lipids, leading to the accumulation of MDA. It has been reported that Met induces the production of superoxide radicals in the brain, which react with nitric oxide to form peroxynitrite. This peroxynitrite oxidizes antioxidant enzymes like SOD and catalase, leading to decreased enzyme activities. Our results showed that ALA treatment reduced adverse effects of Met on the anxiety, pain, and recover SOD and CAT activities in Met-treated groups. ALA is a potent free radical scavenger and reduces ROS in both lipid and aqueous phases. Additionally, it potentiates antioxidant capacity of cells by increasing intracellular glutathione or vitamins (C and E) recycling. On the other hand, ALA inhibits the activation of pro-inflammatory molecules and stimulates the production of anti-inflammatory cytokines such as IL-10. Additionally, ALA increases nerve growth factor expression which, in turn upregulates SOD and catalase genes expression. Importantly, it elevates cholinergic neurotransmission, which, in turn, increases SOD and CAT genes expression [20]. Therefore, it seems that ALA prevents oxidative damage partly by elevating SOD and CAT activity or by reducing inflammatory factors in the PAG.
Our results showed that the administration of Met led to the development of hyperalgesia and anxiety-like behaviors. Additionally, Met administration was associated with increased levels of MDA, a marker of oxidative stress, and decreased activities of CAT and SOD, which are important antioxidant enzymes, specifically in the PAG. However, the treatment with ALA proved to be effective in mitigating these adverse effects. ALA treatment resulted in decreased MDA levels, increased CAT and SOD activities, and importantly, prevented the development of hyperalgesia and anxiety-like behaviors induced by Met. These findings suggest that ALA possesses antioxidant properties and exerts protective effects against the neurotoxicity induced by Met in the PAG. The ability of ALA to restore the redox balance and enhance the activities of antioxidant enzymes may contribute to its beneficial effects in attenuating the behavioral and biochemical alterations associated with Met administration.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This work was supported by Islamic Azad University, Damghan Branch, Iran (grant 14560).
The authors declare that they have no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kargar HM, et al. "Alpha-Lipoic Acid Decreases Pain, Anxiety-Like Behaviors and Oxidative Stress in the Periaquadactal Gray Matter in Methamphetamine-Treated Rats". J Neurosci Neuropharmaco, 2024,10(1), 1-5.
Received: 21-Oct-2023, Manuscript No. NCOA-23-117908; Editor assigned: 24-Oct-2023, Pre QC No. NCOA-23-117908 (PQ); Reviewed: 07-Nov-2023, QC No. NCOA-23-117908; Revised: 17-Jan-2024, Manuscript No. NCOA-23-117908 (R); Published: 24-Jan-2024, DOI: 10.35248/NCOA.24.10(1).104
Copyright: © 2024 Kargar HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.