Research Article - (2022) Volume 11, Issue 1
Introduction: Intra-articular steroid injections are the first-line antirheumatic drugs of oligoarticular onset juvenile arthritis (oligo-JA). Despite significant advances in treatment (anti-TNF, block IL6) the choice of DMARDs for the pediatric chronic joint disease remains relevant. This study aimed to search for candidate biomarkers influencing efficacy treatment by early intra-articular steroid injections in children with oligo-JA.
Patients and Methods: Efficacy of early isolated intra-articular injections of triamcinolone acetonide was analyzed in 120 children with oligoarticular onset Juvenile Arthritis (JA).
Results and Discussion: 42 children (all girls; 35%) were achieved remission JA after one or two isolated intra-articular injections (is- IAI) of Triamcinolone Acetonide (TA). 78 children (54% girls; 11% boys) didn’t achieve inactive JA after two and more is-IAI of TA. There were no determined relationships between clinical, laboratory signs and efficacy is-IAI of TA in children with oligo-JA. The analysis revealed a correlation between a short phase of beneficial effect after is-IAI of TA and risk of activity disease (with an inactive phase of arthritis less than 3 months, the risk activity was OR=2.09, p<0.001; with an inactive phase less than 2 months OR=8.9, p <0.001).
Conclusion: TA is an efficacy treatment in children with oligoarticular JA. There are no biomarkers for the prediction of poor treatment response in oligo-JA to early steroid injections. But a short phase of beneficial effect after is-IAI of TA may be a sign of risk activity disease.
Oligoarticular juvenile arthritis • Local steroid therapy • Triamcinolone acetonide
Oligoarticular juvenile arthritis • Local steroid therapy • Triamcinolone acetonide
Juvenile Arthritis (JA; JIA) is a chronic childhood inflammatory disease-causing erosive arthritis in children, often progressing to disability. Increased incidence of JA worldwide in the closing decades of the 20th century, joint contractures at an early stage of the disease and a high incidence of disability determine the relevance of improving the methods of diagnosis and treatment of rheumatic pathology. Juvenile arthritis is an autoimmune disorder characterized by chronic inflammation of the synovium of one or more joints and an outcome like arthrosis-arthritis. The rapid achievement of inactive arthritis and remission is considered a priority goal of drug therapy for JIA. An intra-articular steroid injection may quickie reduce inflammation and synovitis. Despite significant advances in the treatment of disease, the choice of first-line antirheumatic drugs for oligoarticular onset juvenile arthritis remains relevant. Pediatric rheumatologists have no consensus on the best modality for treatment to this day [1-6].
Juvenile Arthritis (JA, JIA) comprises a group of heterogeneous forms of arthritis characterized by an unknown cause persistent joint inflammation lasting longer than 6 weeks. Typical classic forms of JIA (systemic, oligo, poly, psoria, enthesopathy and others) are well known. About half of all children with JIA have chronic oligoarthritis (oligoarticular JA; oligo-JA). Oligoarticular JA can be persistent (this type of JIA affects fewer than five joints throughout the disease), extended (after the initial 6-month period the total number of affected joints exceeds four), or have a short-term (abortive) course. Oligo-JA is predominantly asymmetrical involves lower-extremity large joints arthritis, such as the knee and ankle joint. The hip joint is rarely affected. Typically oligo-JA affects pre-school girls (peak at 2-4 years). Besides the knee and ankle joints, the elbow, wrist or one or two small joints of the hand and (or) foot can be remotely involved. More rarely clinical course of oligoarticular JA maybe like symmetric arthritis or monoarthritis. Uveitis risk is found to be higher in ANA-positive oligo-JA children. Low laboratory inflammatory markers are features of oligoarticular JA [7-16].
Currently, the majority of pediatric rheumatologists adhere to the stepwise scheme of treatment in oligo-JA. Amplify selectively antiinflammatory therapy is dictated by the persistence of “active arthritis”, progressive joint damage or uveitis (exclude systemic arthritis). This treatment approaches for JIA provides quickly achievement of inactive arthritis and realizes control of the disease course. The strategy of treating JIA without systemic manifestations can be represented by three main directions: the first and most often use "therapy ahead of the disease", the second - "treatment de facto" i.e. treatment flare-up of arthritis; and the third-orthopedic surgeon curing. Despite the aggressive JIA, several relatively unresolved issues remain. It is well known that some children with uveitis-negative of oligo-JA have experienced a controlling course of the disease without systemic anti-rheumatic drugs (DMARS). The opinions of pediatric rheumatologists about the treatment of this subtype of oligoarticular JA are in most cases divided [17-20].
This study aimed to search for candidate biomarkers influencing efficacy treatment by early intra-articular steroid injections in children with oligo-JA.
In a retrospective study, 120 children (89% girls) aged median 4.2 (1.6-7.6) years with oligoarticular onset JIA without extra-articular manifestations (oligo-JA) who did not receive DMARDs were monitored (figure 1). All children have met ILAR criteria (ILAR 1997; 2001; the Edmonton revision 2004). Triamcinolone acetonide was administered intra-articularly at a dose of 20 mg-40 mg with an injection interval of 3,6,12 months, depending on the activity of the disease. The maximum allowable number of consecutive isolated intra-articular injections (is-IAI) was 3-4. All children were divided into two groups: active/inactive arthritis based on the efficacy of local corticosteroid treatment. All children who did not achieve remission oligo-JA for is-IAI were treated by DMARDs. The features of the clinical, instrumental and laboratory pictures of onset disease, the performance of the articular syndrome and the efficacy of intra-articular therapy of corticosteroid drugs were studied. The average follow-up was 48 (38, 62) months. This study was carried out at the rheumatology department of H. Turner National Medical Research Center for Children's Orthopedics and Trauma Surgery. A retrospective analysis of efficacy treatment by is-IAI in 92 children with oligoarticular juvenile arthritis was published earlier [21].
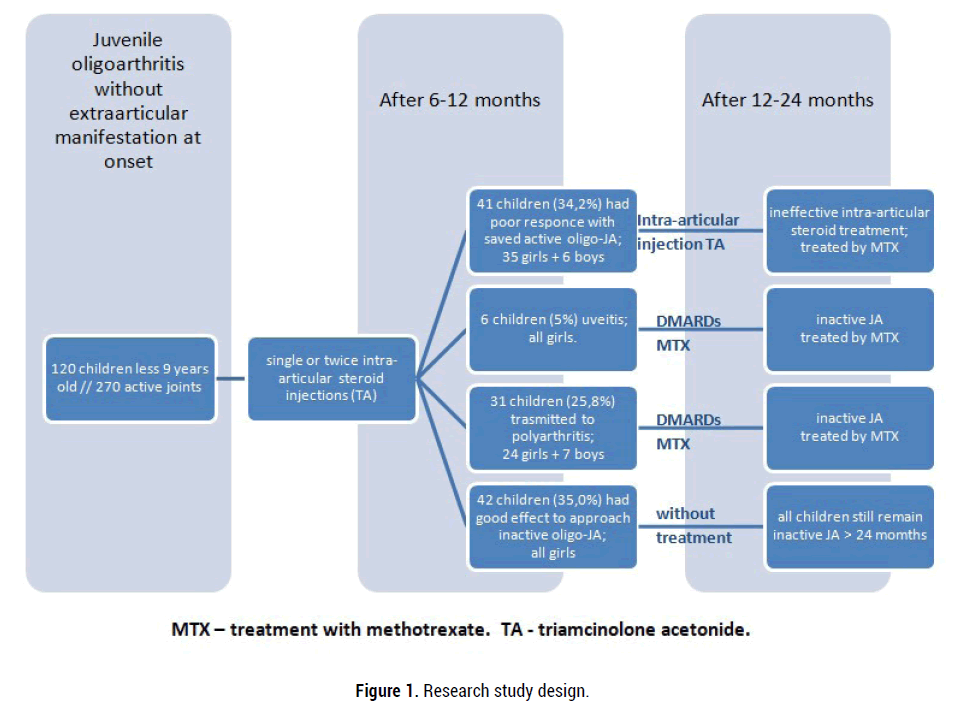
Figure 1: Research study design.
Achievement of inactive disease and clinical remission of JA were evaluated according to Wallace criteria Assessment of X-ray changes in juvenile arthritis was performed using a modified scoring method by Steinbrocker Efficacy of treatment of the oligo-JIA and ongoing disease activity was evaluated by a clinical 10 joint Juvenile Arthritis Disease Activity Score (cJADAS10) Digital data were statistically processed using a software package Microsoft Еxcel and Statistica 6.0. The distribution pattern of variants was determined by Kolmogorov-Smirnov criterion, total variance equation was controlled by F-test. The results were represented as a median (Me) with an interquartile range (25Q-75Q) . To determine the significance of differences between the groups we used nonparametric dispersion analysis (ANOVA) by Kruska-Wallis test (for independent groups) Wilcoxon criterion (for dependent groups). The correlation between the parameters under study was analysed using Spearman rank correlation analysis (r). In all statistical analysis procedures, p=0.05 was taken for a critical significance level of the null statistical hypothesis.
Immunological research was carried out in the laboratory of H. Turner National Medical Research Center for Children's Orthopedics and Trauma Surgery. and the laboratory of autoimmune diseases of St. Petersburg State Medical University. The serum and synovial fluid concentration of tumour necrosis factor-alpha/TNF-α were determined using an enzymelinked immunosorbent assay (Elisa-TNF-alfa; Vector-Best, Russia). The serum and synovial fluid concentration of interleukin 6/IL6 were determined by the electrochemiluminescence immunoassay method on Cobas E411 (Roche, Switzerland). Antinuclear factor/ANF was detected by indirect immunofluorescence technique using HEp-2 epithelial cells as the substrate.
A total of 270 active joints were injected with triamcinolone acetonide: knees-196 injections, ankles-68, elbow-6. Single or twice is-IAI of triamcinolone was administered in 64,2% (77/120) of children, three or more times-35,8% (43/120). Only one joint was treated in 45% (54/120) of children, in 38.3% (46/120) two different joints, in 16.7% (20/120) three or more joints.
42 children (35%; all girls) were achieved remission oligo-JA after is-IAI of triamcinolone acetonide with a mean of 2 (1;2) injections per joint (inactive arthritis>24 months). 21.7% (26/120) of these children had monoarthritis, 8.3% (10/120)-asymmetrical oligoarthritis and other 5% (6/120)-symmetrical oligoarthritis lower-extremity (Table 1). The mean interval between two consecutive is-IAI was 8 (6;12) months. Other children (65%) did not achieve inactive oligo-JA after is-IAI of triamcinolone acetonide with a mean of 3 (2;4) injections per joint. 25.8% of these children (31/120; 24 girls and 7 boys) had transmitted polyarthritis after two is IAI, 5% (6/120; all girls)-onset uveitis, other 34.2% (41/120; 35 girls and 6 boys) had flamed joints (active oligo-JA) after more than three is-IAI. The mean interval between the first two consecutive injections was (5, 4;7) months and other injections-2 (2;3) months. All children who did not achieve remission oligo-JA for is-IAI were treated by DMARDs.
| Groups of childrenSubtypes (%) | Value and frequency | P-value | |
|---|---|---|---|
| first group (n=42) | second group (n=78) |
||
| Monoarthritis | 26♀ (62.0%) | 24♀ (30.8%) | p<0.01 |
| 0♂ (0%) | 5♂ (6.4%) | p>0.05 | |
| Asymmetrical oligoarthritis | 10♀ (23.8%) | 21♀ (26.9%) | p>0.05 |
| Symmetrical oligoarthritis | 6♀(14.2%) | 18♀ (23.1%) | p>0.05 |
| ♂ (0%) | 6♂ (7.7%) | p>0.05 | |
| Psoriatic oligoarthritis | 0♀ (0%) | 2♀ (2.5%) | p>0.05 |
| 0♂ (0%) | 2♂(2.5%) | p>0.05 | |
| Note: ♀ - girls, ♂ - boys | |||
Table 1. Clinical manifestation at onset oligo-JA subtype in children.
The main treatment-related complications were post-injection reversible (transient) manifestations of local hypopigmentated atrophic skin. Complication rates of knee and ankle joints did not exceed 10%. No other complications were noted. Statistical analyses were performed to determine the relationships between clinical, laboratory signs and efficacy is-IAI of triamcinolone acetonide. Measures included the number of swollen or tender joints (active joint counts) and cJADAS10; biological inflammatory markers (Erythrocyte Sedimentation Rate ESR), C-Reactive Protein (CRP), serum and the synovial fluid level of Interleukin 6 (IL6) and umour necrosis factor alfa (TNF-α); ( Autoimmunity Titer of Antinuclear Factor (ANF). According to the results of this study differences in level ESR, CRP, IL6 and TNF-α serum, IL6 and TNF-α synovial were obtained inchildrenat the onset of inactive and active oligo-JA (Tables 2,3 and figure 2).
| Groups of children Parameters, Me (25Q;75Q) | Value and frequency | P-value | |
|---|---|---|---|
| first group | second group | ||
| (n=42) | (n=78) | ||
| Girls, abs (%) | 42 (100%) | 65 (83.3%) | p<0.01 |
| Age of onset JA, years*1 | 2 (2;3) | 3 (2;7) | p>0.05 |
| Number of active joints, abs*2 | 1 (1;2) | 1 (1;2) | p>0.05 |
| cJADAS10 | 10 (8;12) | 11 (8;14) | p>0.05 |
| ESR, mm/h | 14 (6;28) | 22 (16;48) | p>0.05 |
| CRP, mg/l | 3,0 (1,4;6,2) | 4,4 (3,2;13,6) | p>0.05 |
| Hemoglobin, g/l | 114 (110;128) | 112 (108;124) | p>0.05 |
| Leucocytes, 109/l | 8,0 (6,8;10,6) | 7,4 (6,2;10,8) | p>0.05 |
| Trombocytes, 109/l | 442 (408;486) | 438 (412;468) | p>0.05 |
| Gamma globulin, % | 21,6 (19,2;22,0) | 22,4 (20,4;23,8) | p>0.05 |
| Interleukin 6 serum (IL6), pg/ml | 4,2 (2,4;7,0) | 6,8 (4,8;12,2) | p>0.05 |
| TNF-α serum, pg/ml | 0,74 (0,2;1,2) | 0,6 (0,1;1,4) | p>0.05 |
| ANF ≥ 1/160, abs (%) | 34 (81%) | 63 (80.8%) | p>0.05 |
| Note: *1: age of children at the onset JIA; *2: number of active joints at the onset JIA; JADAS: Juvenile Arthritis Disease Activity Score; ESR: erythrocytes sedimentation rates; СRP: С-Reactive Protein; TNF-α serum: Tumour Necrosis Factor alpha of serum; ANF: Antinuclear Factor.. | |||
Table 2. Clinical and laboratory features at onset oligo-JA subtype in children.
| Groups of children Parameters, Me [25Q;75Q] | Value and frequency | P-value | |
|---|---|---|---|
| first group (n=32) | second group (n=60) | ||
| Cytosis, 109/l | 4,3 (3,4;7) | 4,4 (3,8;6,6) | p>0.05 |
| lymphocytes, % | 54 (32;66) | 32 (24;52) | p>0.05 |
| Neutrophiles % | 18 (12;38) | 40 (6;54) | p>0.05 |
| Monocytes % | 16 (8;22) | 14 (9;20) | p>0.05 |
| Synoviocytes, % | 2 (0;8) | 1 (0;4) | p>0.05 |
| Ragocytes, % | 3 (2;4) | 3 (2;7) | p>0.05 |
| Interleukin 6 synovial (IL6), pg/ml | 2120 (710;4268) | 3234 (806;12044) | p<0.01 |
| TNF-α synovial, pg/ml | 3,4 (2,4;4,2) | 1,6 (0,8;4,8) | p<0.01 |
| Note: TNF-α synovial: Tumor necrosis factor alpha of synovial fluid; Ragocytes: cells (macrophages, neutrophils) containing large granules: phagolysosomes, including immune complexes, various immunoglobulins, rheumatoid factor. | |||
Table 3. Cytological analysis, levels TNF-α and IL6 of synovial fluid at onset oligo-JA in children.
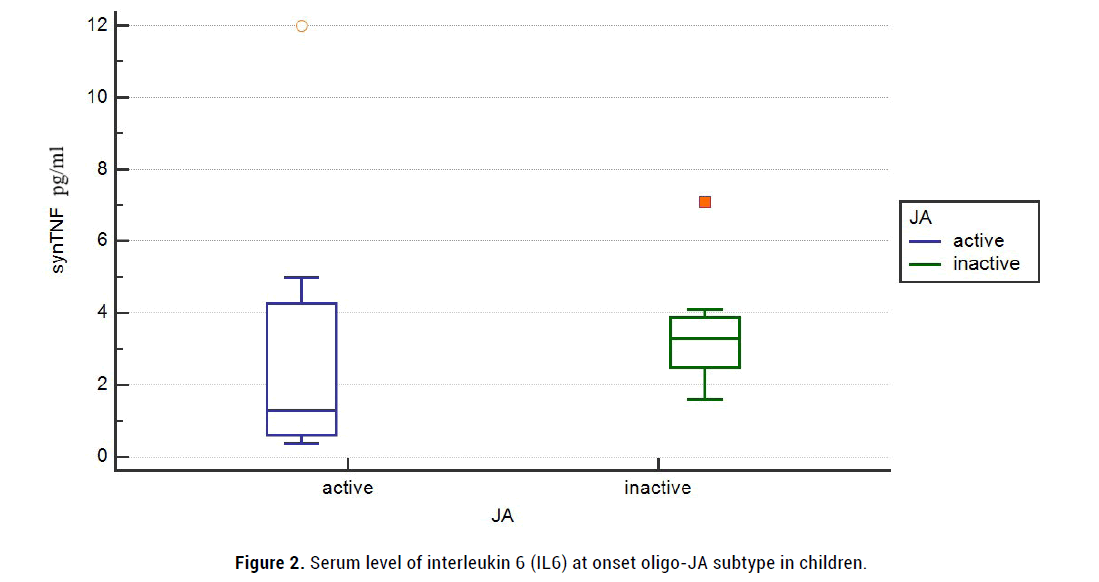
Figure 2: Serum level of interleukin 6 (IL6) at onset oligo-JA subtype in children.
But efficacy is-IAI of triamcinolone acetonide was not associated significantly with several active joints of onset oligo-JA, cJADAS10, serum level of CRP mg/ml, ESR mm/h, IL6 pg/ml and TNF-α pg/ml, the titer of ANF. The mean inflamed synovial fluid of IL6 (synIL6) levels varied at onset and were high in children with active oligo-JA (2120 (710; 4268) vs 3234 (806; 12044) pg/ml). Paradox, but mean level of synovial TNF-α (synTNF) were higher at onset in children with inactive oligo-JA (3,4 (2,4; 4,2) pg/ml vs 1,6 (0,8; 4,8) pg/ml; (figure 3- 4).
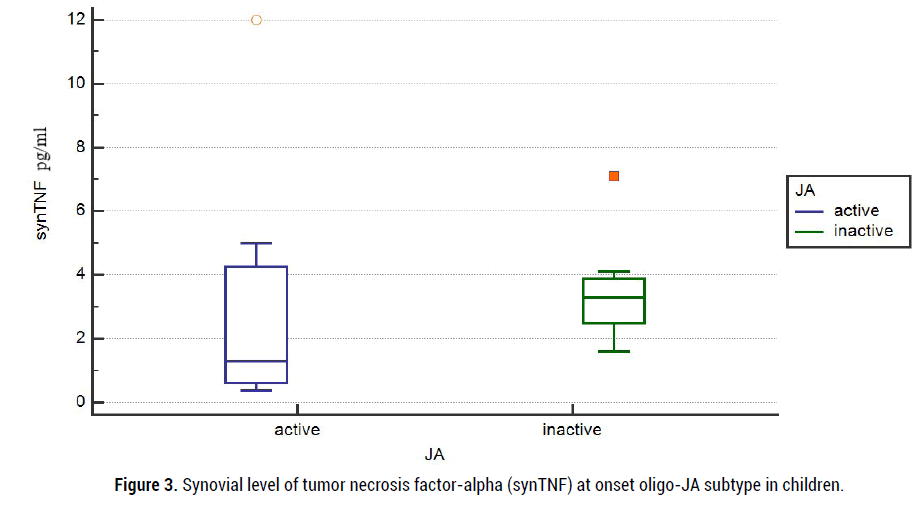
Figure 3: Synovial level of tumor necrosis factor-alpha (synTNF) at onset oligo-JA subtype in children.
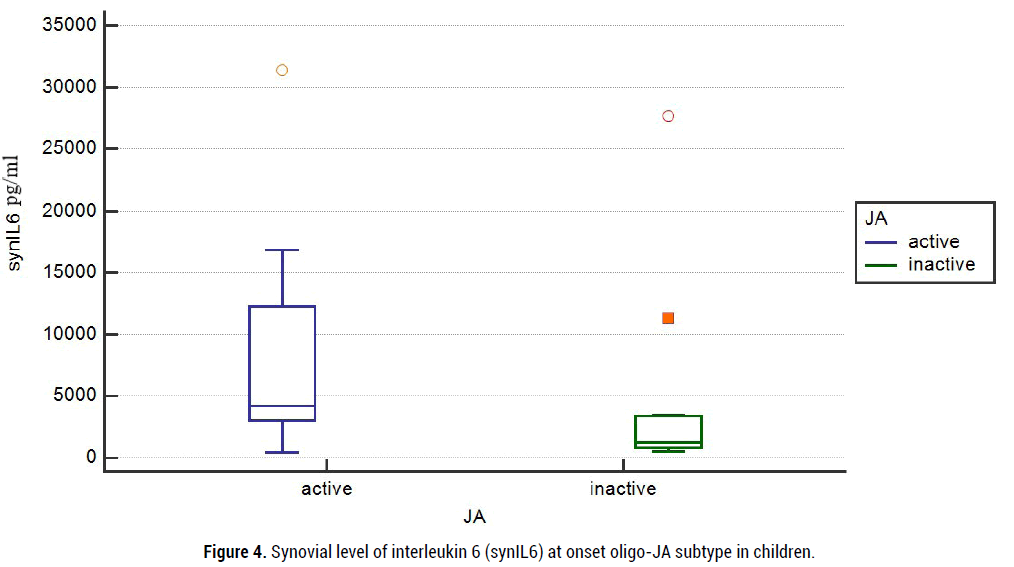
Figure 4: Synovial level of interleukin 6 (synIL6) at onset oligo-JA subtype in children.
An attempt was made to search for models of predicting the efficacy of non-systemic steroid therapy in children. At the first glance, girls with monoarthritis were predominated in the group with inactive oligo-JA (26/62.0%-achieved remission vs. 24/30.8%-active JA; p<0,01, c2=2,8). However, statistical analysis did not revealed a correlation between monoarthritis and the inactive stage after is-IAI (R2=0.158, T=1.23, OR=4.01, 95% CI 0.57-27.69, p=0.227). A significant positive correlation was not found in the multiple linear regression analysis between efficacy is-IAI and age-onset JA, articular lesion and inflammatory laboratory parameters (Table 4).
| Parameters | Estimate | Standard error | P-value |
|---|---|---|---|
| Until 2 age of onset JIA | 2,14,055 | 1,38,001 | p=0.1209 |
| 2-4 age of onset JIA | 1,79,825 | 1,26,700 | p=0.1558 |
| 4-6 age of onset JIA | 0,80445 | 1,27,093 | p=0.5268 |
| 6-8 age of onset JIA | 2,34,254 | 1,30,479 | p=0.0726 |
| Sex - boy/girls | -21,39,576 | 80,06,41,242 | p=0.9979 |
| Monoarthritis at onset | 0,83360 | 0,46415 | p=0.0725 |
| Asymmetrical oligoarthritis at onset | -0,34484 | 0,5085 | p=0.4977 |
| Symmetrical oligoarthritis at onset | -0,89994 | 0,62423 | p=0.1494 |
| Psoriatic oligoarthritis at onset | 00000 | 00000 | 00000 |
| Joint - knee at onset | 0,09867 | 0,1119 | p=0.3807 |
| Joint - ankle at onset | 0,97601 | 0,69056 | p=0.1575 |
| Level ANF ≥ 1/160 ≤ | 0,32493 | 0,43004 | p=0.4499 |
| Level ESR, mm/h | -0,003841 | 0,009596 | p=0.8638 |
| Level CRP, mg/l | -0,01390 | 0,02447 | p=0.6246 |
| Level IL6 serum, pg/ml | -0,03193 | 0,02386 | p=0.2557 |
| Level TNF-α serum, pg/ml | 0,1144 | 0,06898 | p=0.1507 |
| Level IL6 synovial, pg/ml | -0,000009239 | 0,00000764 | p=0.2913 |
| Level TNF-α synovial, pg/ml | -0,02611 | 0,01474 | p=0.1882 |
| Good effect of is-IAI > 6 months | 0,7745 | 0,04702 | p<0.001 |
| Good effect of is-IAI 3-5 months | 0,1311 | 0,09810 | p=0.1312 |
| Good effect of is-IAI < 3 months | -0,5849 | 0,1249 | p<0.001 |
| Good effect of is-IAI < 2 months | -0,5849 | 0,1151 | p<0.001 |
Table 4. Poisson regression modelling of inactive arthritis by efficacy steroid intra-articular injection in children with onset oligo-JA.
The analysis revealed a correlation between a short phase of beneficial effect after is-IAI of triamcinolone acetonide and risk of activity disease (with an inactive phase of arthritis less than 3 months, the risk activity was OR=2.09, p<0.001; with an inactive phase less than 2 months - OR= 8.9, p <0.001; (Table 5).
| Coefficient of determination R2 | Standard error | T-test | Odds ratio OR | 95% CI odds ratio | Significance level, p | |
|---|---|---|---|---|---|---|
| More than 6 months | -0,7745 | 0,04702 | -16,470 | 5,09 | 0,956 - 1,000 | p<0.001 |
| 3-5 months | 0,1311 | 0,09810 | 1,337 | 2,03 | 0,393- 4,643 | p=0.1312 |
| Less than 3 months | 0,5849 | 0,1249 | 4,683 | 2,09 | 0,534 - 0,749 | p<0.001 |
| Less than 2 months | 0,5849 | 0,1151 | 5,081 | 8,9 | 0,680 - 0,868 | p<0.001 |
Table 5. Poisson regression modelling of inactive arthritis in children with onset oligo-JA based on the phase of beneficial effect after is-IAI of TA.
We limited our analysis to studies the efficacy of intra-articular injections of long-acting corticosteroids (triamcinolone acetonide) in children with oligoarticular-onset of JIA. The main purpose of the research was to find possible predictors (clinical, instrumental or laboratory) of early ineffective of is-IAI in children with oligo-JA. To meet the objectives of the study, the approach to diagnosis and management of JIA in one medical centre was used. Moreover, the parents had a great interest in the course of the study. It is no secret that the parents do not agree to early aggressive therapy by methotrexate. This position is based on the amount of information available on the web about the side effects of methotrexate, conclusions of non-rheumatological specialists and fear of lifelong treatment . In addition, single or the small number of affected joints, low activity of the disease leave hope for a favourable outcome or misdiagnosed JIA (in the opinion of most parents). In this situation, the parents of the child usually agree to the next intra-articular injection of triamcinolone into the inflamed joint [22-24].
According to national (Russian) clinical guidelines, intra-articular glucocorticoids are recommended as the first-line therapy for oligoarticular JIA. Currently, there are small studies about the is-IAI efficacy of oligoarticular JA in children in the Russian Federation. A metaanalysis of summary data from various trials showed the efficacy of intraarticular corticosteroid injections in JIA. In Europe and the USA, the longacting steroids most commonly used in JIA for intra-articular treatment are Triamcinolone Hexacetonide (TH). TH has been used in Britain and the USA for intra-articular injections since 1967. In Pediatric rheumatology, the earliest published study using intra-articular injections comes from Petty et al. in 1986 [25-29]. In the Russian Federation for intra-articular joint injection in JIA use Triamcinolone Acetonide (TA).
Triamcinolone Acetonide (TA) is a synthetic glucocorticoid with antiinflammatory properties. The pharmaceutical composition of a sustained release suspension of triamcinolone provides a long-term influence on the inflamed synovium (TA 40mg/ml). Triamcinolone has been shown to decrease the expression of pro-inflammatory cytokine (TNF-α, IL-1β, IL- 6), chemokines and growth factors (VEGF, IGF, PDGF, CSF-1), block the proteolytic activity of matrix metalloproteinases (MMP-2, MMP-3, MMP- 9) and reduce migration of inflammatory cell in synovial space. A decrease in the proliferative capacity of synoviocytes is associated with a reduction of NF-κB transcriptional activity [30-32].
Most multicenter studies have demonstrated the safety and efficacy mode of therapy intra-articular injections of triamcinolone hexacetonide. Additional studies have demonstrated superior efficacy of triamcinolone hexacetonide compared to triamcinolone acetonide. Triamcinolone acetonide was positioned as an alternative form acceptable for intraarticular injections. The long-term efficacy of local therapy with triamcinolone hexacetonide/acetonide in juvenile arthritis was also reflected in more recent studies, but triamcinolone hexacetonide was preferred [33-39].
In the present study, the efficacy of non-systemic corticosteroid therapy was related due to several determining factors. The first injection of triamcinolone at the earlier stage of oligoarticular JA, preliminary dilution of TA to 10 mg/ml-15 mg/ml and a special post-injection regimen were the main factors. Efficacy and optimal timing of is-IAI in children with JIA should be strictly limited to 6-12 months after the onset of the disease. Following injections can be done in inflamed joints without signs of disease progression. The preliminary dilution of triamcinolone and active joint range of motion improves the corticosteroid drug penetration deeper into the inflamed synovial membrane. Reduce axial loads on the lower extremity decrease risks of aseptic necrosis and contribute to prolonged therapeutic effect. Long-term effects of intra-articular administration of triamcinolone were related to anti-inflammatory, anti-proliferative, immunosuppressive and vasoconstrictive actions on the synovial membrane. Triamcinolone is the corticosteroid with the slowest joint clearance and the most potent in producing synovial atrophy. Scalding mechanism of triamcinolone on inflamed synovial tissue associated with vasoconstriction of blood vessels of subsynovial layers and the return of cells sensitivity to pro-apoptotic factors [40-43].
For all 120 children with oligoarticular onset JIA without extraarticular manifestations, the original trial completed the 7-year follow-up. The present study was shown the efficacy and safety of the “therapeutic step” of non-systemic corticosteroid therapy with triamcinolone acetonide in children with oligoarthicular-onset JA. About a third of children achieve remission of arthritis > 2 years after is-IAI (all girls). There are no biomarkers for the prediction of poor treatment response in oligo-JA to early steroid injections. The difference obtained from the results of laboratory characteristics of children at the onset of inactive and active oligo-JA may indicate a measure of the activity of the disease. But we didn’t find a correlation between laboratory marker and efficacy of is-IAI. The short phase of beneficial effect after is-IAI of TA in our study was only one sensitive indicator of risk activity disease. For determined sensitive synovial IL6 and TNF-α to activity JIA is necessary to introduce data on low, middle and high concentrations of them. Comparatively higher levels of synovial TNF-α at onset in children with inactive olgo-JA will need further study. In addition, boys with oligoarticular onset juvenile arthritis may be considered potentially ineffective for local steroid therapy.
TA is an efficacy and safety treatment in children with oligoarticular juvenile arthritis. Using local corticosteroid drugs in children with oligo-JA like a tool of defined sensitivity to arthritis makes it possible to assess of aggressive course of the disease. Despite the era of biological therapy is- IAI in children has not lost relevance and efficacy.
Not declared.
The study was approved by the local ethics committee at H.Turner National Medical Research Center for Сhildren's Orthopedics and Trauma Surgery
(Protocol No. 1 dated 01.20.2014).
Google Scholar Cross Ref
Google Scholar Cross Ref
Google Scholar Cross Ref
Google Scholar Cross Ref
Google Scholar Cross Ref
Google Scholar Cross Ref
Google Scholar Cross Ref
Citation: Kozhevnikov AN, Pozdeeva NA, Nikitin MS, et al. Steroid Intra-Articular Injections in Children with Oligoarticular Onset Juvenile Arthritis: 7 Years Follow-up Study. J Arthritis, 2022, 11(1), 001-006.
Received: 24-Dec-2021, Manuscript No. 50537; Editor assigned: 26-Dec-2021, Pre QC No. 50537; Reviewed: 15-Jan-2021, QC No. 50537; Revised: 23-Jan-2021, Manuscript No. 50537; Published: 27-Jan-2022, DOI: 10.4172/2167-7921.2022.11.055
Copyright: 2022 Aleksei KN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.