Research Article - (2015) Volume 4, Issue 4
Objective: to observe the effects of Xinfeng Capsule on adjuvant arthritis rat model of pulmonary function, B, T cell immunity. Methods 40 rats were randomly divided into normal, model, Tripterygium Glycosides tablet and XFC group. Except the normal group, the other rat’s right rear paw intradermal injection of Freund’s complete adjuvant induced arthritis in 0.1 mL. Inflammation induced 19 d after administration, the normal group, the model groups were given physiological saline, rats in the treatment group were given of Xinfeng Capsule (2.4 g/kg), Tripterygium Glycosides tablet (10 mg/kg), once a day, continuous administration of 30D. Joint inflammation in rats was observed performance, flow cytometry detection of peripheral blood B, T lymphocyte attenuator (BTLA), regulatory T cells (Treg) and surface markers of forkhead transcription factor (FoxP3) expression was detected by Western blotting, synovial membrane, lung tissue BTLA, FoxP3. Results compared with the normal group, model group of rat paw swelling degree, arthritis index increased; pulmonary function, reduce the expression levels of peripheral blood BTLA, Tregs and BTLA in pulmonary tissue, synovium, FoxP3 (P <0.05 or P <0.01). While the XFC group rat paw swelling and arthritis index lower than the model group, pulmonary function, BTLA, Tregs and FoxP3 increased (P <0.05 or P <0.01); Xinfeng capsule group is better than control group (P <0.05) medicine Tripterygium wilfordii. Conclusion of Xinfeng capsule may be through upregulation of BTLA, Tregs, and FoxP3 expression, reduce the stimulation of inflammatory mediators in the lung tissue and improve the lung function of rats with adjuvant arthritis.
Keywords: B, T lymphocyte attenuator; Regulatory T cells; Adjuvant arthritis; Pulmonary function; Xinfeng capsule
Rheumatoid arthritis (RA) is a kind of unknown etiology of chronic, inflammatory synovitis based system diseases. Pathological RA arthritis synovial lining cells, mainly hyperplasia of interstitial infiltration of inflammatory cells, and the formation of micro angiogenesis, pannus tissue and cartilage and bone destruction of the. RA in addition to joint lesions outside can also invade other tissue and organ of [1]. Lung tissue containing blood vessels and connective tissue, rich therefore, lungs are more vulnerable and secondary pulmonary lesions [2]. RA pulmonary lesions occupy the most important status in the extra articular lesions. The study found that the risk of pulmonary involvement in RA disease for more than 8 years of up to 60.8% [3]. RA pulmonary disease early symptoms of respiratory system and imaging manifestations of mild or atypical, and the changes of pulmonary function in the early clinical manifestation and chest radiographic abnormalities of respiratory system before [4]. Therefore, a systematic and in-depth observation of the pulmonary lesions has become a hot research of RA pulmonary function injury. RA damage of lung function in addition to direct stimulation and inflammation, one of the important links of immune system disorders is the disease. T cell immunity in particular regulatory T cells (Treg) and B, T lymphocyte attenuator (BTLA) play an important role of connective tissue diseases [5]. Previous study found that [6], RA in patients with joint swelling, pain, morning stiffness and other symptoms, lung tissue showed interstitial fibrosis changes, a direct manifestation of lung function decreased. At the same time reduced pulmonary function in peripheral blood of patients with Tregs, the surface markers FoxP3 and BTLA reduction. For further verification of Tregs and BTLA in RA mediated by reduced lung function process, through the adjuvant copy of adjuvant arthritis (AA) rat model, to observe the pulmonary function of AA rats and peripheral blood Tregs, BTLA changes, the detection of expression of FoxP3 and BTLA in lung tissue of the synovial Tregs and surface marker, and investigate the role of B and T cell immunity in reduced lung function in RA. Traditional Chinese medicine plays an important role in the treatment of RA and extra articular lesions [7]. Previous studies showed that [8,9], with Replenishing Qi to invigorate the spleen, removing dampness and dredging collaterals, painkillers effect of Xinfeng Capsule.Chinese medicine Xinfeng Capsule, which consists of Huangqi (Astragalus membranaceus), Yiyiren (Semen coicis), and Wugong (centipede), can not only improve symptoms and signs of joints in patients with RA, but also obviously improve their pulmonary function. Xinfeng Capsule can improve RA symptoms such as joint swelling, joint pain, and morning stiffness, lower the indexes of joint pain and joint tenderness reduce blood sedimentation, C-reactive protein, anti-CCP and rheumatoid factor, etc. XFC can obviously improve the pulmonary function in patients with RA parameters such as forced vital capacity (VC), forced expiratory volume in 1 second (FEV1), forced respiration rate of 1 second (FEV1 / FVC), forced vital capacity 25% of maximum expiratory flow (FEV25), forced vital capacity 50% of maximum expiratory flow (FEV50), forced vital capacity 75% of maximum expiratory flow (FEF75), the largest tidal midexpiratory flow (MMF), maximum expiratory flow (PEF), etc. XFC can improve the symptoms and signs of pulmonary such as cough, sputum, chest tightness, wheezing, short of breath. Xinfeng capsule improve the pulmonary function of AA rats by adjusting the peripheral blood level of regulatory T cells, inhibiting Th1 cells activation, prompting Th1 cells drift to Th2 cells, keeping cellular immune in an inhibitive condition, and making the Th1/Th2 in balance, minimizing the harm of inflammatory cells on lung tissue, and promoting the ventilation function of lung tissue. Mechanism of improving RA pulmonary function for the further research of Xinfeng capsule. This paper observed the effects of Xinfeng Capsule on BTLA, Tregs and FoxP3 in AA rats, to explore the Xinfeng capsule and improve lung function is by regulating B and T cell immunity in AA rats.
Experimental animals and drug
40 male SD rats of clean grade, rats aged 8~10 months, body weight (220 + 20) g, provided by experimental animal center of Anhui Province, licensenumber: SYXK (Anhui) 2013-0004.
Breeding of clean grade standard. Xinfeng Capsule: 0.4 g/ particle, provide preparation center, the First Affiliated Hospital of Anhui traditional Chinese Medicine University batch 2012100407; tripterygium glycosides tablet: 10mg/ sheet, production, Medical University of Shanghai red flag pharmaceutical factory batch number: 2011101504.
Main reagent and instrument
Freund’s complete adjuvant (USA): Sigma company, batch number: 113k6572; phycoerythrin (PE) BTLA kit labeled America: BD company, batch number: 100521; fluorescein isothiocyanate (FITC) labeled mouse anti CD4 monoclonal antibody, phycoerythrin cyan pigment dye 5 (PE-Cy5) labeled rat anti CD25 monoclonal antibody, PE labeled mouse anti FoxP3 America: eBioscience company, batch number: 11-0040-81, 11-0040-45, 11-0040-76; Rabbit anti BTLA, anti FoxP3, mouse anti-rat-actin antibodies: Santa Cruz USA beta company, batch number sc-66821, sc-13066, sc-8432. Animal lung function testing instrument: Beijing bestlab Technology Co., Ltd production (AniRes2003). Electrophoresis: USA Amersham Company (EPS-301). Gel image analyzer: American Bio-RAD Company (Gel Doc XR). Microscope: Germany LEICA company (DMLB), a system of automatic microphotographic: Japan OLYMPUS (CX31-32C02). Flow cytometry American: Beckman Coulter Company (Epics XL).
The model copy, grouping, administration: The study protocol was approved by the Ethics Committee of First Affiliated Hospital, Anhui University of Chinese Medicine (Hefei, China). 40 rats in random comparison table were randomly divided into normal control group 10 rats with adjuvant arthritis group 30, except the normal group, to the right foot of each rat paw intradermal injection of Freund’s complete adjuvant induced arthritis in 0.1mL, copied into the AA model, based on the improved model of replication method, strengthen the immunity of [10] in the 7d in the rat tail injection FCA 0.05mL. Nineteenth days after inflammation, 30 adjuvant arthritis rats in the model group according to the random comparison table were randomly divided into 3 groups: model group, Tripterygium wilfordii group, XFC group, started the day administration, through the human body surface area calculation of rats dose, equivalent to 10 times the clinical adult dosage. The normal and model groups were given normal saline (1 g/kg), Ra Masato (10 mg/kg) group, XFC group (2.4 g/kg) were given the Ra Masato multi glycoside tablet and Xinfeng Capsule suspension. Once a day, continuous use 30 days later the rats were killed, the following indexes were detected.
Calculation of paw swelling and arthritis index (AI) in rats: Paw volume was measured every 3 days. Paw swelling (E) was calculated [9] using the following formula:E (%) = (Vt – Vn)/Vn × 100% .Where Vn and Vt represent the volume of the paw before and after modeling, respectively. Systemic and joint disease caused by inflammation was recorded every 3 days. The AI was classified using a five-scale method [11]: 0, no swelling; 1 point, swelling on the joint of the little toe; 2 points: swelling on the metatarsal phalange joint and foot; 3 points, swelling on the hindpaw below ankle swelling; 4 points, swelling on the hindpaw and including ankle swelling. The sum of points for each rat was calculated, and the highest possible score was 12 points.
Evaluation of pulmonary function: Everal parameters of pulmonary function were evaluated. These included average expiratory flow, which was calculated by dividing FVC by the value for forced expiratory flow in one second (FEV1) and multiplying by 100%. Further, 25%, 50%, and 75% of the vital capacity of the peak expiratory flow (FEV25, FEF50 and FEF75, respectively) were calculated. Peak expiratory flow (PEF) was also assessed. These measurements were obtained using the pulmonary function test apparatus for small animals thirty days after administration. Rats were anesthetized with 10% chloral hydrate (0.35 mL/100 g, i.p.). Tracheotomy and endotracheal intubation were then carried out. The rats were then put into an airtight box. The ventilator tube was connected to the mechanical ventilation apparatus to collect pulmonary function. In this setup, external pressure caused deep inspiration. Computer software was used to measure each indicator automatically.
Observation of lung tissue pathology morphological: Rats were intraperitoneally injected with 10% chloral hydrate solution (0.30 ml/100g) anesthesia after treatment for d30. Open the abdominal cavity isolated trachea and double lung tissue. The pathological changes of lung tissue were observed with the naked eye, the lung tissue fixed in 4% paraformaldehyde. After in order to dehydration, transparent, waxing, embedding, slicing, HE staining and radiography.
Detection of BTLA, CD4+ CD25+ FoxP3+ Treg: Blood 100uL adding PE labeled mouse anti BTLA antibody of 10 mu L, room temperature evades the light reaction of 30 min; 2 mL hemolytic agent at room temperature for lysis of red blood cells 10 min; PBS washing, centrifugal 5 min (1500 r/min), Kami Kiyo; each specimen add 1 g/L of paraformaldehyde 500uL for the detection of BTLA lymphocyte percentage after accounting for flow cytometry. Treg determination: take on the sample tubes, each tube to join CD4 20uL, CD25 10uL; room temperature dark incubation of 15-20min; FoxP3 20uL, room temperature dark incubation of 25-30 min. PBS washing, centrifugation 5 min (1500 r/min), Kami Kiyo; each sample was added 1 g/L paraformaldehyde 500uL, using CD4+ CD25+FoxP3+ Treg flow cytometry was used to detect the ratio of CD4+ in T cells.
Synovial, lung tissue BTLA, detection of FoxP3 protein in lung tissue: Extraction of total protein with protein extraction kit, according to the 25 g/ holes on the capillary electrophoresis. Constant pressure 90 V electrophoresis to the separation gel upper, constant pressure 140 V electrophoresis to the separation of glue bottom part; in the constant current of 270 mA transmembrane 50min. Closed with 5% skim milk powder 2 h, add 1 to 1000 dilution of BTLA, FoxP3 monoclonal antibody, shaker at room temperature were incubated for 2 h, phosphate buffer (PBST) 3 times washing, each 10 min. Add 1 to 3000 dilution of Goat anti-mouse IgG-HRP, table were incubated for 1 h, 3 PBST washing, each 10 min. ECL reagent and exposure were scanned by scanner. Using BANDSCAN software with the analysis of the article, the calculation of band gray value, with BTLA, FoxP3/ beta -actin gray value as BTLA, FoxP3 protein expression in lung of synovial and relative quantity.
Statistical analyses: Continuous variables are expressed as mean ± standard deviation. All samples were tested to ascertain if they followed a normal distribution. Data comparison among groups was performed using ANOVA. Comparison between groups was carried out using the independent samples t-test. SPSS Version 11.5 (SPSS Inc., Chicago, IL, USA) was used for data analyses. P<0.05 was considered significant.
Effects of Xinfeng Capsule on joint inflammation expression, pulmonary function
Toes swelling degree: adjuvant induced arthritis, model group of rat paw swelling degree, arthritis index increased gradually, and the pulmonary function parameters of FEV1, FEF50, FEF75, PEF decreased. Drugs for the treatment of 30d, Xinfeng capsule group and Tripterygium wilfordii group rat paw swelling and arthritis index lower than the model group, the lung function increased (P <0.05 or P <0.01). The comparison between the XFC group and Tripterygium wilfordii group, no significant difference between the performance of joint inflammation (P >0.05); while the XFC group pulmonary function parameter FEF75 is Tripterygium wilfordii group increased (P <0.05) Tables 1 and 2.
| Group | Toes swelling degree (%) | Arthritis index (points) | ||
| Before inflammation | After administration | After 12d inflammation | After 30d administration | |
| Normal | 1.60 ± 0.37 | 9.87 ± 1.93 | 0.11 ± 0.09 | 0.12 ± 0.10 |
| Model | 1.53 ± 0.48 | 20.1 ± 5.72** | 1.39 ± 0.53** | 8.32 ± 2.18** |
| Tripterygium wilfordii | 1.51 ± 0.39 | 11.5 ± 3.36△△ | 1.46 ± 0.47** | 5.66 ± 2.02△△ |
| Xinfeng Capsule | 1.59 ± 0.46 | 15.2 ± 4.74△ | 1.41 ± 0.52** | 6.22 ± 1.92△ |
| Notes: Compared with the NC group,** P <0.01. Compared with the MC,△△P <0.01,△P <0.05. | ||||
Table 1: Comparison of toe swelling and arthritis index in rats (n=10, 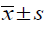 )
)
| Group | pulmonary function parameters | ||||
| FEV1 | FEF25 | FEF50 | FEF75 | PEF | |
| Normal | 68.42 ± 6.93 | 46.28 ± 4.73 | 43.72 ± 5.43 | 35.62 ± 5.26 | 56.81 ± 5.36 |
| Model | 57.43 ± 8.85** | 43.83 ± 5.26 | 37.46 ± 4.35* | 22.72 ± 5.16** | 45.11 ± 5.35** |
| Tripterygium wilfordii | 64.62 ± 5.31*△ | 46.56 ± 4.73 | 39.73 ± 6.11 | 28.43 ± 5.35*△▲ | 49.26 ± 6.82*△ |
| Xinfeng Capsule | 65.74 ± 5.96*△ | 45.42 ± 5.48 | 39.89 ± 3.87 | 37.36 ± 4.52△△ | 46.36 ± 5.36*△ |
Table 2: Comparison of pulmonary function parameters of rats (n=10, 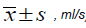 , ml/s)
, ml/s)
Effect of Xinfeng Capsule on lung tissue and the morphological
Observation with light microscope, the normal group rats lung tissue structure is clear, the alveolar structure of regular. The structure of alveolar lung tissue of model group is not structured, alveolar atrophy or disappear, partly in the lung of substantive changes, visible infiltration of inflammatory cells in the lung interstitium. Tripterygium wilfordii group alveolar structure clear, more standard, part of alveolar cavity shrinking, deformation, pulmonary septal thickening. XFC group alveolar structure is regular, part of alveolar cavity atrophy. Electron microscopy revealed that the structure of cells, alveolar epithelial cells in rats of normal group II integrity, cell number is more, cytoplasm rich in mitochondria and no swelling, deformation; the number of type II alveolar epithelial cells of the rats in the model group decreased, membrane structure is not complete, the boundary is not clear, in the cytoplasm, mitochondria swelling, degeneration and reduced. The proliferation of fibroblast interstitial lung. The structure of alveolar epithelial cells in rats of group of Tripterygium wilfordii are mostly intact, mitochondrial swelling, deformation is not obvious, some plate emptying phenomenon of lamellar bodies, collagen fiber hyperplasia is reduced, eosinophil infiltration. The structure of the alveolar epithelial cell type II basic integrity of Xinfeng capsule group, rough endoplasmic reticulum is abundant cytoplasm, mitochondria largely intact, a few mild mitochondrial swelling and deformation Figures 1 and 2.
Effects of Xinfeng Capsule on BTLA, Tregs
Flow cytometry results show, the expression of peripheral blood BTLA, model group rats, CD4+ CD25+ Treg CD4+ CD25+ FoxP3+ Treg lower than the normal group (P <0.05 or P <0.01). After drug treatment, Xinfeng capsule group and Tripterygium wilfordii group BTLA, increase the expression of Tregs (P <0.05). Compared with the control medicine Tripterygium wilfordii group, XFC group CD4+ CD25+ FoxP3+ Treg increased (P <0.05) Table 3, Figures 3 and 4.
| Group | BTLA | CD4+CD25+ Treg | CD4+CD25+ FoxP3+Treg |
| Normal | 53.37 ± 5.06 | 12.11 ± 3.37 | 4.96 ± 1.06 |
| Model | 42.74 ± 4.63** | 7.98 ± 2.68** | 3.14 ± 0.97* |
| Tripterygium wilfordii | 45.25 ± 5.72△* | 9.52 ± 2.28△* | 3.21 ± 0.88▲ |
| Xinfeng Capsule | 44.93 ± 5.63△* | 10.03 ± 3.74△* | 4.75 ± 0.96△ |
Table 3: Comparison of BTLA, Tregs in peripheral blood of rats (n=10, 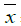 ±s, %)
±s, %)
Effect of Xinfeng Capsule on BTLA, FoxP3 protein in synovium and lung tissue
Western blotting results show that, compared with the normal group, the model group rats synovial, lung tissue BTLA, FoxP3 protein decreased (P <0.05 or P <0.01). Treatment, compared with model group, XFC group rat synovium and lung tissue BTLA, FoxP3 protein level (P <0.05 or P <0.01). Compared with the positive control medicine Tripterygium wilfordii group, XFC group BTLA, FoxP3 protein level (P <0.05) Figures 5 and 6.
RA induced lung injury is T lymphocyte control inflammatory reaction. BTLA, in the T cell immunity of Treg is closely correlated with RA. BTLA and Treg may be a common expression of regulatory T cells, thus affecting the RA levels of lung function. CD4+CD25+ Treg are a subtype of CD4+T cell, activation of CD4+T cells is a key link in the pathogenesis of RA. CD4+CD25+Treg cells can inhibit RA secretion of inflammatory cells. BTLA is specifically expressed in T, B cells; BTLA can negatively regulate T, B cell activation, proliferation and Treg expression of [12,13].
Treg has immune energy and immunosuppression function of [14,15]. Treg dependent inhibition of T cell activation by cell contact, maintaining self-tolerance [14,16]. FoxP3 is the surface of Treg specific markers Treg, FoxP3 on growth development, function, and immunological tolerance plays an important role in [17,18]. Found that rats appeared a series of inflammation in adjuvant induced arthritis can be from the results of this study, lead to joint inflammatory cell infiltration. With the gradual progress of chronic inflammation, pulmonary function changes, the performance for the decline in lung function. Further investigation revealed that the peripheral blood BTLA, AA, rat Tregs expression decreased, at the same time, synovium and lung tissue BTLA, FoxP3 also reduced accordingly. Description T, immune dysfunction of B cell adjuvant induced arthritis may be involved in the process of decline in lung function. T, B cells can produce a large number of proinflammatory cytokines such as interleukin, resulting in organ injury of different degree in lung tissue, including. BTLA, RA Tregs may participate in the process of immune regulation. BTLA mainly by inhibiting the over activation of T cell to regulate cellular immune. CD4+T cells of primary and secondary immune response, BTLA crosslinked T cell antigen receptor can inhibit T cell activation [19,20]. The abnormal expression of BTLA protein can be expressed molecular CD25 markers of activation effect of T cells, thus affecting the differentiation of Tregs. While Tregs expression disorder will also affect BTLA expression. BTLA, Treg through the synergistic regulation [21,22], release inhibition of inflammatory molecules, thereby reducing the inflammatory injury of medium on RA lung tissue organ. The study found that increased BTLA expression in patients with [23,24], RA ould secrete inflammatory factor specific downregulation of immune inflammation, reduce the level of RA, thereby regulating the immune balance, relieve the progress of RA extremely extra articular lesions. The results also showed that AA reduced pulmonary function in rat peripheral blood, lung, synovial tissue BTLA decreased expression level. The results of research and Shang et al. Similar to [5] RA pathogenesis in traditional Chinese medicine is characterized by “the spleen wet Sheng, deficiency”. Damage of spleen qi and body fluid metabolism disorders, fluid retention, poly and phlegm, drink, effect of dispersing and descending the lung and the emergence of asthma sputum more clinical manifestation. The treatment of the use of “spleen and stomach, nourishing the day after tomorrow, acute symptoms, phlegm dampness” therapy. Invigorating spleen for eliminating dampness Tongluo Xinfeng Capsule in Chinese medicine the whole regulation as the basic principle, can joint symptoms improved significantly in AA rats and improve lung function in [25]. The study found that, after drug treatment, Xinfeng Capsule could obviously increase the expression of rat peripheral blood BTLA, Tregs and synovial, lung tissue BTLA, FoxP3 protein, and significantly better than the control drug group. This study shows that of Xinfeng capsule may be through upregulation of BTLA, Tregs, FoxP3 expression, and thus inhibit joint and AA rats lung tissue inflammation, reduce airway inflammation. The composition of Xinfeng Capsule “astragalus root, coix seed, Lei Gongteng, centipede etc.. Radix astragali containing astragalus polysaccharides and glycosides, can regulate T cell immune function; saponins, polysaccharides can increase the expression of body CD4+CD25+ Treg; lung injury astragalus polysaccharides alleviated bleomycin induced. Astragalus can regulate BTLA, Treg imbalance, the inhibition of immune complex and collagen fibers deposition, improvement in [26] synovium and lung tissue injury of joint. Coix seed extract contains Coixenolide, Coixenolide, Coixenolide significantly inhibited the secretion of neutrophils, lymphocytes, but also inhibit the formation of pulmonary fibrosis. Triptolide can inhibit the abnormal immune response in patients with RA, the study found that [27,28], Tripterygium wilfordii upregulation of Tregs and FoxP3 expression in [29], Tripterygium wilfordii monomer can reduce the degree of alveolitis and pulmonary fibrosis, pulmonary fibrosis with anti [30] effect of certain. The centipede extract can induce cell apoptosis, at the same time, the centipede containing histamine like substance, can significantly increase the pulmonary microvascular opening number, and has obvious anti-inflammatory effect of [31,32]. Xinfeng Capsule by astragalus root, coix seed, Lei Gongteng, centipedes compatibility, to combine attack, while tonifying, supplementation and attack effect. On the one hand, RA can regulate the immune function, on the other hand, can also prevent the Tripterygium wilfordii immunosuppression caused by excessive immune deficiency state. A compatibility characteristic of this attack supplementation plays a role in regulating the balance, the curative effect is better than the single use of Tripterygium wilfordii. While this study of Xinfeng Capsule superior efficacy of Tripterygium wilfordii also show that compatibility characteristics of the.
In summary, Xinfeng capsule may be through upregulation of BTLA, Tregs, FoxP3 expression and regulation of B, T cell immunity, inhibiting immune complex and infiltration of inflammatory cells, reduce the synovium, pulmonary vascular permeability, thereby improving joint symptoms and pulmonary function.
The authors express their gratitude to Mr Wen Hu, Ke Cheng, Qinglin Li for their excellent technical assistance. The authors thank Lei Liu, Yuanyuan Cheng, Yunxia Feng for assistance. This work was supported by grants from the National Natural Science Foundation Project (81173211), National Key Discipline of Traditional Chinese Medicine Project for Bi Diseases [Administration of Traditional Chinese Medieine , China (2009) NO. 30], National Administration of Traditional Scientific Research Special Foundation of China (2004-2005LP27), Anhui Traditional Chinese Medicine applied basic research and development of provincial experimental Room Program (Section [2008] 150), Anhui Education Department Natural Science Key Research Program (KJ2008A165).