Review Article - (2014) Volume 4, Issue 2
Though the theories on the reasons why humans need to sleep vary, there is no disagreement that sleep is essential to our wellbeing and survival. External and internal physiological parameters dictate our own individual body “clocks,” or circadian rhythms. Typically, this clock runs very close to a 24-hour cycle of sleep and wakefulness. However, for some individuals, this clock can be slightly “off.” This article will discuss what creates internal circadian rhythms and the major types of circadian rhythm disorders, with an overview of diagnosis and treatment options.
Humans spend 1/3 of our lives sleeping, yet sleep is still a great mystery surrounded by many theories. It is estimated that millions of people around the world suffer from sleep or wakefulness disorders. Sleep Medicine is a relatively new field, which has made astounding progress in the past 30 years; however, the importance of sleep is underemphasized and healthcare professionals under recognize sleep disorders.
Sleep complaints are very common in the general patient population, yet physician education regarding sleep disorders is historically poor, due to limited sleep medicine education in undergraduate medical training and in residency programs [1,2]. In fact, a survey of medical schools in the US and Canada revealed that only about three hours is dedicated to sleep medicine education [3]. Currently, none of the primary specialties (internal medicine, surgery, family medicine, and obstetrics-gynecology) have topics on sleep disorders in their required curriculum [4].
Primary care physicians are often the first to evaluate patients for any reason, and play a critical role in the recognition of sleep issues and the subsequent diagnosis, treatment, and/or referral to a sleep medicine specialist. This article serves to provide an update on sleep theories and the sleep cycle, and provide an overview of a subset of sleep disorders called circadian rhythm sleep disorders. The goal is to provide the primary care provider with information and references to current CRSD research.
According to the National Institutes of Health “Sleep is a required activity, not an option. Even though the precise functions of sleep remain a mystery, sleep is important for normal motor and cognitive function. Sleep actually appears to be required for survival. Rats deprived of sleep will die within two to three weeks, a time frame similar to death due to starvation [5].”
A recent and widely accepted definition of sleep, according to the American Academy of Sleep Medicine is: “Sleep is much more than a gentle ‘pause’ from your daily activities. It is an active state that helps maintain and renew your mental and physical health. Behind the curtain of sleep at night, your brain controls important functions that set the stage for the next day. Muscles are repaired. Breathing, heart rate, blood pressure, and hormone levels are regulated. New information is processed, and memories are formed. Sleep is essential for your health and is the most valuable part of your day. After a good night's sleep, you will feel, think, and perform your best [6].”
Several widely accepted, but still debated theories of sleep function include [7]:
Memory consolidation
Energy conservation: energy expenditure and oxygen consumption are reduced during sleep
Brain restoration: an increase in growth hormone release during sleep promotes brain and body growth
Protective behavioral adaptation: periods of darkness avoid exposure to predators
Immune function regulation: immune function deteriorates during sleep as shown by experimental data
Sleep can be split into two major phases, Non-rapid Eye Movement (NREM) and Rapid Eye Movement (REM), both which cycle 4-6 times per night in a normal sleepers [8]. Slow wave sleep or “deep sleep” dominates the first third of the sleep period (as represented by stages 3 and 4 in the Figure 1 below), and REM sleep dominates the last third of the sleep period, with the REM episodes increasing in duration throughout the night [9]. The first REM episode occurs about 90 minutes after a person falls asleep, and alternates with NREM every 90-100 minutes for the remainder of the sleep period as seen in the Figure 1 [8]. Wakefulness should account for less than 5% of the night [9].
The sleep-wake cycle is regulated by two biological processes, which aim to balance each other. This is widely accepted as the “2 process model” of sleep regulation [11]. Process S is a sleep/wake dependent homeostatic process and Process C is controlled by a circadian oscillator. These two processes together generate the timing of sleep and wake.
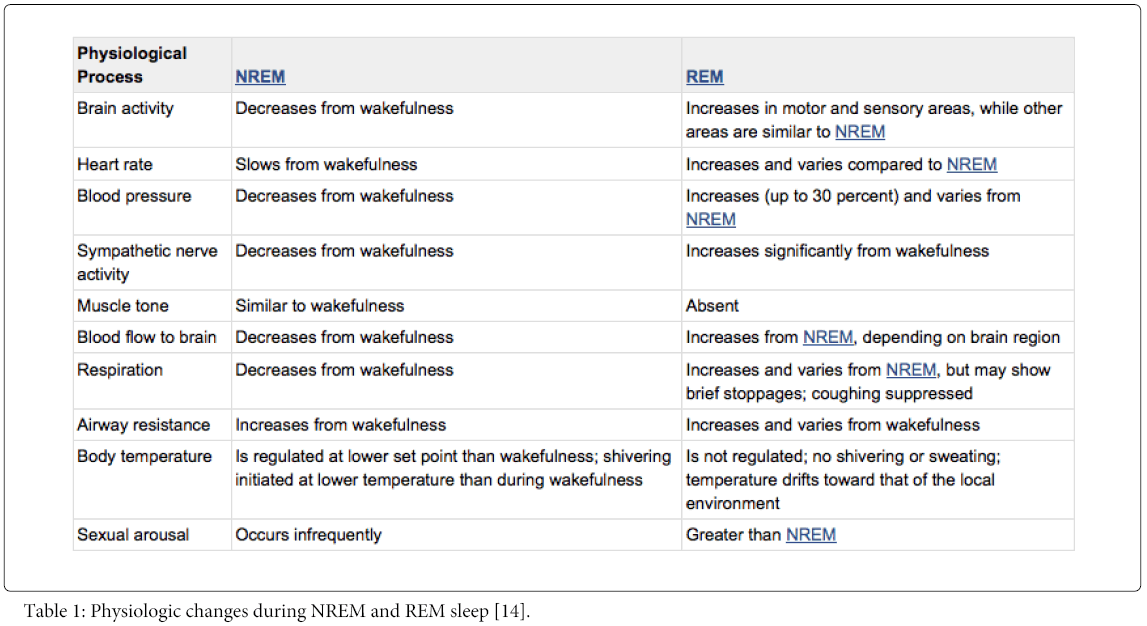
Sleep architecture is the term used to describe the basic structural organization of normal sleep [12]. NREM sleep usually accounts for 75% to 80% of sleep and REM sleep usually accounts for 20% to 25% of sleep [9]. NREM sleep can be separated into several additional stages, each representing a continuum of deepening sleep [12]. NREM, REM, and Wakefulness each are completely different physiological states. The physiology of NREM and REM sleep is summarized in the following Table 1.
Our daily lives are a cycle of physiologic processes and behaviors driven by circadian rhythms, which are about (circa) one day (diem) [8]. The sleep-wake cycle is our most obvious circadian rhythm. The human circadian rhythm is generated by the suprachiasmatic nucleus (SCN) of the hypothalamus, as shown in the Figure 2 [13].The SCN acts as an internal biological clock, with an essential role of promoting wakefulness during the day and sleep during the night. Alignment of the desired sleep/wake schedule (perceived and required) and the timing of the internal biological clock are necessary for “optimal sleep quality”[15].
Figure 2: “The biological clock is located within the suprachiasmatic nucleus in the brain” [16].
Circadian rhythms in humans are most influenced by environmental factors or “cues”. These environmental factors help synchronize the sleep/wake cycle and other physical factors, such as core body temperature, and circadian timing related hormone secretion with a typical 24 hour period [9]. Markers and time cues help to synchronize or “entrain” our circadian clock. In the absence of time cues or “free running”, the human clock actually persists at just over 24 hours [17]. Markers and time cues that are environmental stimuli are called zeitgebers [18]. The most important zeitgeber is the solar day (light and darkness), and typically, the human circadian rhythm entrains to the solar day [19]. Other zeitgebers include: feeding, ambient temperature (in some individuals), activity and arousal states, social cues [19], and others.
According to the International Classification of Sleep Disorders, circadian rhythm sleep disorder is defined as “a persistent or recurrent pattern of sleep disturbance” due primarily to “alterations of the circadian timing system”, or “misalignment between the endogenous circadian rhythm and exogenous factors that affect the timing or duration of sleep” [7].In other words, when a person’s internally driven sleep/wake cycle is regularly disrupted and does not align with their optimal sleep/wake cycle, a circadian rhythm sleep disorder may be present. Internal and/or external factors (e.g. social or environmental) are likely contributors. Symptoms include insomnia and/or excessive daytime sleepiness, as well as associated “impairment of functioning”, such as “social, occupational, or other”. The degree a person is affected by circadian misalignment varies. Some people are very sensitive to its effects, while some experience little to no effects Figure 3 [20].
Delayed Sleep Phase Disorder (DSPD) is characterized by regular delays in a person’s ability to fall asleep. This delay is usually more than two hours later than socially acceptable or desired bedtimes.1 DSPD patients are considered “owls” or “evening types”. Clinical features of DSPD include: difficulty initiating sleep before 2-6 a.m. and preferred wake times between 10 am and 1 pm [15]. If the person follows their preferred sleep schedule, their sleep is often of normal length and quality. This is often not the case due to work schedule and social obligations. In these cases, patients often seek treatment because of insomnia, excessive sleepiness with difficulty waking in the morning, and functional impairments – interfering with work, school, and social function [21]. DSPD most commonly affects adolescents [15].
Diagnostic criteria often includes actigraphy for at least seven days and/or a sleep diary demonstrating a regular delay of sleep. [7] Polysomnographic findings are essentially normal for age in DSPD when the recording is performed during the patient’s preferred delayed sleep time. When the recording is done when the patient is following a conventional sleep schedule, polysomnographic findings will show prolonged sleep latency and decreased total sleep time [22]. Treatments for DSPD may include chronotheraphy, timed bright light therapy, melatonin, and other approaches aimed at resetting the circadian clock. Melatonin is a nutritional supplement and is not regulated by the Food and Drug Administration (FDA), but is approved for use in treating sleep disorders. Studies involving melatonin are limited by the small number of participants, but studies have shown that administering melatonin (5 mg) 5 hours before sleep onset resulted in advancing sleep onset time [23-25].
Advanced Sleep Phase Disorder (ASPD) is characterized by sleep and wake times that are several hours earlier than the desired or socially acceptable norms. The sleep period is usually stable. ASPD patients will typically and involuntarily fall asleep between 6 and 9 p.m. and wake between 2 to 5 am [26]. Individuals with ASPD are described often as “larks” or “morning types”. Symptoms of ASPD typically include sleep maintenance insomnia, early morning awakenings, and late afternoon or early evening sleepiness ASPD [15].prevalence is much lower than DSPD. ASPD is mostly associated with age and middle age and older adults are the most commonly affected age groups [22].
In addition to the clinical history, actigraphy and/or sleep diaries are useful in the evaluation and diagnosis of ASPD. These should be recorded for at least two weeks. Polysomnography is not required for the diagnosis of ASPD [15]. If polysomnography is performed during normal sleep time in patients with ASPD, sleep architecture and duration will be normal, but if performed at more conventional times, NREM sleep duration, particularly slow wave sleep, is reduced [27]. Treatment approaches for ASPD include chronotheraphy, timed bright light therapy in the early evening (between 7:00 pm and 9:00pm), avoiding light early in the morning, and pharmacotherapy with sedative-hypnotics or administration of melatonin in the early morning [28]. However, there is little evidence to support the effectiveness of pharmacological therapy [29]. Depression and other causes of sleep maintenance insomnia must be ruled out before confirming the diagnosis of ASPD [30].
A well-defined circadian sleep-wake cycle is absent in patients with irregular sleep-wake rhythm. Instead of having a major sleep period each day, patients present with three or more sleep episodes during a 24-hour period, each with varying length. Sleep periods are irregular and fragmented, but total sleep time per 24-hours is usually age normal [31]. Endocrine, body temperature, and other circadian rhythms may also show a loss of diurnal variability [7]. The prevalence of irregular sleep-wake rhythm is unknown, but is estimated to be rare. Irregular sleep-wake rhythm is most often associated with neurological dysfunction, such as brain injury, dementia, and psychomotor retardation in children [22].
Diagnosis includes requires a complaint of insomnia and/or excessive sleepiness associated with irregular sleep episodes in a 24- hour period [26]. Diagnostic tools include continuous monitoring with polysomnography for at least 24-hours or actigraphy for at least two weeks. Recordings typically show disturbed or low-amplitude circadian rhythm with no normal diurnal sleep-wake pattern [32]. It is important to note that poor sleep hygiene, as well as the irregular schedules of shift workers and frequent transmeridian travel can mimic irregular sleep-wake rhythm.
Also known as non-24-hour sleep-wake syndrome, non-entrained type is characterized by a sleep-wake cycle longer than 24-hours. Sleep-wake episodes are delayed to later hours each day – alternating between synchrony and asynchrony with the environmental schedule [33]. Patients will report a progressive delay in the timing of sleep and wake times, and attempts at maintaining a regular sleep-wake schedule can lead to development of excessive daytime sleepiness and insomnia symptoms [15].
The prevalence of non-entrained type is unknown, but it is thought to occur in over half of totally blind people [7]. The circadian clock of the average person is slightly longer than 24-hours, and normal person maintained in an environment devoid of time cues eventually develops a free running rhythm. Circadian rhythms of patients with free running rhythms appear to reflect those of people in time-free environments, which may indicate failure of light/dark entrainment [30]. The decreased or lack of Photic reception seems to explain why free running type is prevalent among blind people and rare in sighted individuals.
Sleep diaries and/or actigraphy recorded for at least two weeks are useful in confirming a free running pattern of sleep and wake [15]. Dim light melatonin onset (DLMO) is also used in advanced practice by sleep specialists to aid in determining if a patient is naturally set on a 24-hour circadian clock, or if they have tendency to have a more free-running internal circadian rhythm [21]. Treatment of irregular sleep-wake rhythm may be difficult and results vary. Treatment options may include sleep hygiene advice, chronotheraphy, bright light therapy, melatonin, or a combination of these [27].
Jet lag is due to the temporary alteration of the external environment relative to the timing of the endogenous circadian rhythm by rapid traveling across time zones [15]. Symptoms include daytime fatigue and sleepiness, insomnia at night, mood changes, concentration problems, general malaise, and gastrointestinal problems [34]. Symptoms usually resolve in several days, once the traveler has adjusted to the new time zone.
Three major factors influence the severity of jet lag, which include: direction of travel, number of time zones crossed, and individual susceptibility. Eastward travel is often more difficult to adjust to than westward travel [22]. This is because eastward travel requires a sleep phase advance, which is more difficult than a sleep delay since the circadian sleep rhythm is longer than 24-hours [27].
Jet lag treatment depends on travel duration and direction. For eastward travel, exposure to light in the morning, even a few days ahead, is useful to help advance the sleep phase in the morning. For westward travel, light should be avoided in the morning. Exposure to light in the evening, even a few days ahead, will help a person adapt their circadian rhythm to the new schedule [27]. Melatonin can be used to minimize jet lab (although not FDA approved for this indication), and the general recommendation is 2-5 mg before bedtime upon arrival, and repeat for up to four days [29,35,36].
Shift work includes non-standard work schedules. These can range from occasional on-call or rotating night shift work to permanent night-shift work. Also included are schedules requiring early awakening from nocturnal sleep. Sleep problems are common complaints among shift workers, but not all shift workers suffer symptoms severe enough to interfere with their work performance and/or social obligations. Shift work coping abilities vary among individuals, and are influenced by work schedule, age, family and personal responsibilities, and diurnal preference [15]. Most night shift workers never fully adapt their internal clocks to their work schedule and many accidents have been attributed to poor performance during night shift work [37].
Shift work disorders may be difficult to evaluate. Characteristics of shift work type include excessive sleepiness relative to work hours and insomnia [22]. Effects on sleep are due to the following factors: [27]
Misalignment between a person’s circadian sleep rhythm and the external environment
Poor sleep quality due to external daytime factors (i.e. light, noise, etc.)
Restriction of sleep because of competition with family, social, and recreational activities
Sleep inertia – especially when naps are taken during work time
Actigraphy, sleep logs and diaries, clinical history, questionnaires, and evaluation of circadian phase markers may be useful in diagnosis. Polysomnography’s primary value may be to rule out other sleep disorders [30]. Clinical management of shift work related sleep disorders is based on two approaches: improving sleep and alertness in the work environment and circadian rhythm realignment[22]. Bright light and melatonin may improve adaptation of a person’s circadian rhythm. Other pharmacological agents, such as sedative-hypnotics may be useful in the clinical management of insomnia due to shift work sleep disorders [27]. Caffeine and other stimulants may be useful, but are limited by side effects and risks of dependency [38].
Patients with acute sleep disturbances may seek treatment with their primary care physician. Primary care physicians should be aware of the complete medical history, as some primary conditions may cause significant changes in sleep, which is important for differential diagnosis. For example, it is common for dementia patients to experience sundowning and subsequently, sleep disturbances with secondary awakenings and confusional wandering at night [39]. It is also becoming more apparent that the quality, not just the quantity, of sleep in children can have direct impacts on their behavior and development. In some cases, learning disorders and attention deficit hyperactivity disorder can be directly attributed to sleep habits [40]. Medications can also cause significant changes in sleep architecture in the brain, thus changing the sleep habits of the patient. Medications can prevent a person from falling asleep, staying asleep, or cause them to wake too early [41].
The American Academy of Sleep Medicine (AASM) recommends a sleep log or diary to assist in the diagnosis of CRSD’s, recommends actigraphy for diagnosis of CRSD’s with the exception of jet lag disorder, and recommends using circadian phase markers as the evaluation tool for Free Running Disorder [42]. Polysomnography is not routinely recommended by the AASM to diagnose CRSD’s, but may be indicated to evaluate for another primary sleep disorder, such as obstructive sleep apnea.
Sleep diaries are helpful in determining a patient’s actual sleep habits; such as bedtime, wake time, total time spent in bed, total time sleeping, awakenings, and activities performed before bedtime. Evaluating these variables as a whole can give the physician an understanding of whether a CRSD is present, or if there may simply be a problem with the patient’s habits. Occasionally, a simple change such as suspending eating and vigorous exercise before bedtime may cause significant positive changes in the patient’s sleep quality. However, sleep diaries are subjective and rely on the patient to be accurate and honest in their reporting. Actigraphy is a more objective option of diagnosis. The actigraph is a device that resembles a wristwatch and is worn by the patient over several days and nights, often up to two weeks or more. The device tracks the periods of activity and rest of the patient. The information is downloaded by the physician and studied for potential CRSD.
If it is determined that diagnosis and treatment by the primary care physician is appropriate, a common treatment is short term pharmaceutical intervention, such as the use of sleep aids to assist in retraining the patient’s normal sleep and wake times (phase shifting). It may also be appropriate for the physician to prescribe natural, over the counter medications such as melatonin to assist with this same type of phase shifting [41]. Another treatment that is becoming more popular because of its noninvasive nature is that of DLMO therapy. This therapy uses exposure to a dim light to activate the suprachiasmatic nucleus (SCN) in the brain to secrete melatonin, thus inducing sleepiness in the patient at the desired time [43].
Patients with conditions that appear to be more chronic in nature, or require more intense investigation and treatment, may need to be referred to a sleep medicine specialist. The sleep specialist will most likely use sleep diaries, actigraphy, and a polysomnogram to determine the true nature of the patient’s complaints. The polysomnogram is an objective diagnostic tool that provides detailed information of a person’s physiology during sleep, thus ruling out any other sleep disorders such as Obstructive Sleep Apnea, Restless Legs Syndrome, or others. If these other disorders are not presented on the polysomnogram, the sleep specialist will then use the combination of data collected from all of the diagnostic measures to determine the best plan of treatment for the patient.
Specific treatment methods for various CRSD’s were discussed previously, but in general, a multimodal approach is most effective for treatment of most CRSD’s. These treatments include timed exposure to light, avoidance of bright light at inappropriate times, scheduled sleep/wake times, and sometimes pharmacologic agents [28]. Light is the most powerful zeitgeber, or entraining agent of the human circadian rhythm, and correctly timed light exposure, sometimes combined with timed administration of melatonin is useful in treating the CRSD’s delayed sleep phase, advanced sleep phase, free-running, irregular sleep wake, jet lag, and shift work [29]. Melatonin is approved by the FDA as a treatment for sleep disorders. The AASM recommends planned sleep schedules, timed light exposure, and timed melatonin administration to treat CRSD’s, indicates hypnotics and stimulants for only shift work disorder and jet lag disorder, and indicates alerting agents (i.e. caffeine and modafinil) as the indicated treatment for shift work disorder [42].
We now live in a 24-hour society that does not always allow our natural circadian rhythms to occur. Demands on our time, artificial light, shift work, travel, and other external and natural occurrences can all be major contributors to potential shifts and misalignment of our 24-hour natural circadian rhythms. If left untreated, circadian rhythm disorders can prove to be not only debilitating, but also extremely dangerous to the person affected and those around them. Primary care physicians being aware of such problems and understanding proper treatment methods can be essential in ensuring continued health and wellbeing of patients.