Research Article - (2019) Volume 11, Issue 4
Background: This study was conducted to determine the effects of vitamin C supplementation with and without endurance physical activity on calcium and parathyroid hormone levels in metabolic syndrome patients.
Methods: In a parallel-randomized, double-blind, placebo-controlled clinical trial, 120 metabolic syndrome patients, was randomly assigned into four groups. Biochemical tests were assessed as baseline and after 12 weeks of intervention. Statistical analysis was performed using SPSS version 20.
Findings: A significant decrease in mean serum calcium levels were seen in participants who received vitamin C or “vitamin C plus 30 minutes/day of physical activity” and “placebo plus 30 minutes/day of physical activity” (p value<0.001). Furthermore, we observed a significant decrease in mean serum levels of parathyroid hormone in participants who received vitamin C or “vitamin C plus 30 minutes/ day of physical activity” compared with other two groups (p value<0.001). Also, taking vitamin C plus 30 minutes/day of physical activity, lowered parathyroid hormone more in compared with only taking vitamin C (- 5.77 ± 7.29 vs. - 4.98 ± 9.54).
Conclusions: We conclude that, adding physical activity to vitamin C supplement might be particularly important in regulation of parathyroid hormone and serum calcium levels in metabolic syndrome patients.
Calcium; Metabolic syndrome; Physical activity; Parathyroid hormone; Vitamin C
MetS: Metabolic Syndrome; IDF: International Diabetes Federation; PA: Physical Activity; PTH: Parathyroid Hormone; SD: Standard Deviation; TG: Triglyceride; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; BMI: Body Mass Index; WC: Waist Circumference; FPG: Fasting Plasma Glucose; TC: Total Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; DLC: Low-Density Lipoprotein Cholesterol.
Metabolic syndrome (MetS) is a cluster of the most dangerous heart attack risk factors: diabetes raised fasting plasma glucose, abdominal obesity, high cholesterol and high blood pressure [1-3]. Based on reports, around 20 to 25 percent of the world’s adult population has the MetS [2]. The underlying causes of the MetS have been suggested as central obesity and insulin resistance [4]. Genetic, environmental factors especially unhealthy dietary habits, aging, proinflammatory state, hormonal changes, and physical inactivity contribute to MetS development [5]. Physical inactivity is one of the main factors for chronic diseases which are estimated to cause 1.9 million deaths, globally [6]. In addition, physical inactivity is considered as the fourth leading risk factor for global mortality causing an estimated 3.2 million annual deaths (6% of global deaths) [7]. Physical activity (PA) decreases the risk for premature death, coronary artery disease, obesity, diabetes, hypertension, cancer and depression thereby lowering medical and medication costs and improving quality of life [8].
In addition, inadequate intake of vitamin C and high levels of parathyroid hormone (PTH) may play important roles in cardiovascular risk, which has been suggested to be associated with MetS [9]. Based on the previous studies, doing regular athletic activities and consuming the antioxidants are among the advised solutions for the MetS [10-12]. Furthermore, antioxidant supplementations especially vitamin C relieve the body of the stress associated with MetS and increase the antioxidant capacity [3]. Moreover, physical exercises on a regular basis promote the health of the individuals and its play a vital role in treating MetS patients [13]. During PA, both skeletal muscle and cardiovascular system being active and PTH are widely expressed [14]. Regarding regular PA controversial studies have been reported [15,16]. Some studies have shown that PA associated with increased serum calcium [16,17] conflict studies also has been reported [18,19] and some author found no changes [20]. The liberty of both PTH and calcitonin is reactive to alteration of free ionized calcium and decreased directly after exercise; whereas that, increased PTH levels after acute PA has been confirmed [21]. Indeed, various studies have shown the contradictory effect of vitamin C on PTH secretion, however some of the investigations showed its beneficial property to lower serum PTH level [22,23]. Vitamin C supplementation is possibly a modality to reduce PTH with less side effects, while several investigations have shown, increasing vitamin C levels by dietary supplementation resulted in reduction of PTH in vitamin C deficient individuals [24,25]. In conclusion, adding PA to vitamin C supplement in MetS patients might be particularly important in regulation of PTH and serum calcium levels and reduce the risk of MetS. Our study was conducted to determine the effects of vitamin C supplementation with and without endurance PA on calcium and PTH levels in MetS patients. To the best of our knowledge, this is the first intervention study, which examined this association among MetS patients.
Study design and participants
This randomized, double-blind, placebo-controlled clinical trial was performed in the Halabja hospital, Kurdistan region of Iraq. The subjects underwent a 12 weeks treatment program (from 01 March 2016 to 23 May 2016). Based on the suggested formulas of this model, the study participants were recruited on the foundation of developed inclusion/exclusion criteria [26]. One hundred-twenty participants were randomly assigned into four groups: Group A received 500 mg/day vitamin C supplements (Morning time), and not participated in 30 minutes/day of endurance PA (n=30); group B received 500 mg/day vitamin C supplements with 30 minutes/day endurance PA (Either at the morning, 7:30 AM or afternoon, after 3:00 PM) (n=30); group C received one placebo of vitamin C/day without endurance PA (n=30); and group D received one placebo of vitamin C/day with 30 minutes/day endurance PA (n=30). All investigators and participants were blinded to the random assignments. Both vitamin C supplements and placebo were obtained from Osweh manufacture Tehran, Iran and was prepared to feature the same shape, odor and size of the supplements.
Participants were asked to use vitamin C supplements and placebos for 12 weeks. Compliance of study participants with the vitamin C supplements was assessed through quantification of serum vitamin C. To ensure maintenance of their habitual diets throughout the study, all participants provided 3-day dietary recalls (One weekend day and 2 weekdays). Then, we converted the reported portion sizes in the dietary records to grams using household measures. All dietary data were based on the average of three dietary recalls. The grams of food intake data were linked with Nutritionist IV software to derive nutrient intake data. To avoid any confounding effects throughout the study, dietary and PA recommendations were given to all participants at the end of the study.
Sample size and sample determination
The sample size was calculated using a previously described formula for parallel clinical trials “n=2[(z1-α/2+z1-β)2.s2]/d2 [26]. In this formula, n is number of participants in each group. For estimating sample size, we considered type one (α) and type two errors (β) of 0.05 and 0.20 (Power=80%) respectively, and fasting plasma glucose levels as a key variable. Based on a previous study, standard deviation (SD) of plasma glucose levels was 8 mg/dL and the difference in mean (d) was considered to be 5 mg/dL. Where α=0.95, β=20%, study power= 80%, d=5, and SD=8” [26].
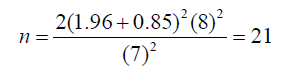
We reached the sample size of 21 subjects for each group. To consider probable dropouts, 30 patients were included in each group. Finally, a total of 120 patients with MetS were included in our study. Participants were distributed into four groups as shown in Figure 1.
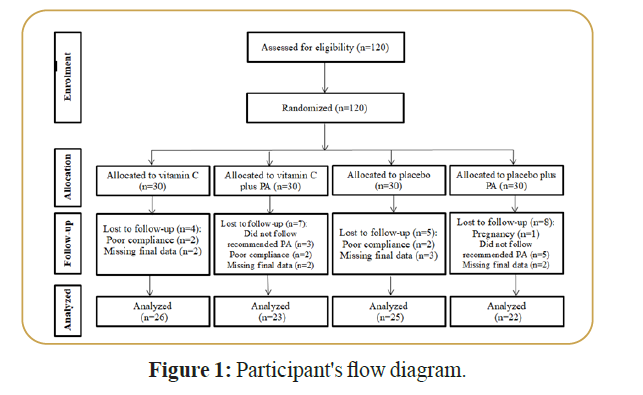
Figure 1. Participant's flow diagram.
Inclusion and exclusion criteria
Eligibility criteria for participants having MetS, according to the International Diabetes Federation (IDF) criteria [2], is the age between 30 and 50 years, both gender, living in Halabja for at least three years. Individuals who took supplements containing vitamins C in the last three years were not included in this study. “Individuals with type I and type II diabetes who were taking oral hypoglycemic agents or injecting insulin, or any medical therapy affecting the result, individuals with heart failure, and those who are suffering from renal problems, also individuals with malabsorption syndrome, pregnancy and lactating mothers were all excluded from the study. The exclusion list included patients with history of bariatric surgery and those who are currently using weight loss medications. We also did not include those with a high triglyceride (TG) levels (more than 400 mg/dl) and those with high systolic blood pressure (SBP) or diastolic blood pressure (DBP) (>140/90 mmHg)” [3].
Data collection
At study baseline a standardized questionnaire [27], was used to collect subjects’ information. In addition, the demographic and medical history variables was obtained and recorded with an interviewbased questionnaire.
Assessment of physical activity
In the present study, PA for all participants was the same. “Two times a week for climbing (Around 2 hours each time) and two times a week for running (Around one hour in the afternoon between 3 to 5 PM), approximately six hours per week all together” [5].
Assessment of anthropometric measures
Height (m), weight (kg), and waist circumference (WC) were measured according to standard [4]. In addition, the body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. To avoid subjective error, all measurements were taken by the same technician [28]. Blood pressure was measured at morning time in the seated position using a standard mercury sphygmomanometer after at least 15 minutes of rest [29].
Biochemical analysis
Fasting blood samples (10 cc) were collected at baseline and 12 weeks of intervention, after 12 hours overnight fasting to quantify serum levels of fasting plasma glucose (FPG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), TG, vitamin C, serum calcium, and PTH levels. The blood samples were taken using the protocol outlined in [30]. Serum Vitamin C was measured by the Model-vidas analyser (Biomerux, Italy) provided by the clinical laboratory, Slemani Hospital, Iraq. FPG was measured on the day of blood collection by enzymatic colorimetric method using glucose oxidase. Serum TC and TG concentration were assayed using enzymatic colorimetric tests with cholesterol esterase and cholesterol oxidase and glycerol phosphate oxidase, respectively, by using standard kits following manufacturers’ protocols. HDL-C was measured after precipitation of the apolipoprotein B containing lipoproteins with phosphotungistic acid. LDL-C was calculated from serum TC, TG and HDL-C based on relevant formula [31]. Finally, serum PTH levels pg/ml were measured using original kits, using perfect plus 400 auto-analyzer (Mindary, UK).
Statistical analysis
All statistical analyses were done using SPSS version 20. Following baseline randomness of allocation of participants in intervention groups by looking at their demographic and medical history characteristics were tested using Chi-square test and ANOVA. After this by student’s t-test and paired t-test the difference of variables under study (Change due to intervention), were investigated by adjusting for effects of baseline measures. p value<0.05 was considered as statistically significant.
Out of the 120 randomized participant at baseline, 47 males and 73 females, overall 24 participants were excluded at the end of the study because of the following reasons: Pregnancy (n=1), did not follow recommended PA (n=8), poor compliance to vitamin C supplements (n=6) and did not complete the study (n=9). Finally, 96 participants completed the study and were included in the final analysis. To be confident that age distribution, gender allocation, marital status, and also the family history of obesity, family history of diabetes mellitus and family history of hypertension of participants in different study groups would not alter the findings after the interventions the distribution of above named characteristics compared in study groups at the baseline. Therefore, as demographic characteristics of the participants were not different across intervention groups, so the analysis of different factors at the end of study does not need to be adjusted for these differences. In the present study, the baseline characteristics of the study participants were shown in Table 1. Our results revealed that, comparison of baseline serum vitamin C levels showed no significant difference among the study groups (p value=0.358). In addition, participants who received vitamin C supplements had higher serum levels of TG compared with those who received placebo or “placebo plus 30 minutes/day of endurance PA” (p value<0.001). Furthermore, participants in the “vitamin C plus 30 minutes/day of endurance PA” group had higher values of HDL-C compared with those in the placebo group (p value=0.004). However, participants in the vitamin C group had lower levels of LDL-C compared with those in the “placebo plus 30 minutes/ day of endurance PA” group (p value=0.027). No significant differences among the study groups in terms of age, gender, marital status, weight, BMI, WC, family history of obesity, family history of diabetes mellitus, family history of hypertension, systolic and diastolic blood pressure, FPG, TC, calcium, and PTH levels (p value>0.05 for all).
| Variables | Group (n=96) | ||||
|---|---|---|---|---|---|
| Vitamin C2 (n=26) |
Vitamin C+PA3 (n=23) |
Placebo4 (n=25) |
Placebo+PA5 (n=22) |
p value6 |
|
| Age (years) | 41.19 ± 5.8 | 40.78 ± 5.8 | 42.60 ± 5.6 | 41.59 ± 6.4 | 0.125 |
| Female (%) | 16.0 (61.5) | 16.0 (69.6) | 12.0 (48.0) | 13.0 (59.1) | 0.71 |
| Married (%) | 22.0 (84.6) | 19.0 (82.6) | 21.0 (84.0) | 17.0 (77.3) | 0.155 |
| Weight (kg) | 81.04 ± 13.1 | 79.72 ± 13.5 | 82.34 ± 13.9 | 74.84 ± 12.6 | 0.467 |
| Body mass index (kg/m2) | 31.96 ± 6.0 | 32.34 ± 5.8 | 32.81 ± 4.3 | 30.13 ± 4.7 | 0.654 |
| Waist circumference (cm) | 108.2 ± 10.4 | 107.8 ± 8.7 | 107.6 ± 9.6 | 111.4 ± 10.2 | 0.563 |
| Family history of obesity (%) | 17.0 (65.4) | 15.0 (65.2) | 19.0 (76.0) | 11.0 (50.0) | 0.427 |
| Family history of diabetes mellitus (%) | 12.0 (46.2) | 8.0 (34.8) | 15.0 (60.0) | 9.0 (40.9) | 0.754 |
| Family history of hypertension (%) | 12.0 (46.2) | 13.0 (56.5) | 15.0 (60.0) | 9.0 (40.9) | 0.326 |
| Systolic blood pressure (mmHg) | 130.1 ± 11.9 | 128.9 ± 11.3 | 125.6 ± 14.5 | 128.6 ± 10.8 | 0.806 |
| Diastolic blood pressure (mmHg) | 81.3 ± 8.3 | 79.3 ± 7.1 | 80.0 ± 6.9 | 82.5 ± 5.9 | 0.523 |
| Fasting plasma glucose (mg/dl) | 106.6 ± 19.9 | 103.9 ± 13.6 | 110.6 ± 17.2 | 106.2 ± 18.1 | 0.635 |
| Total cholesterol (mg/dl) | 174.7 ± 41.5 | 178.2 ± 29.7 | 185.9 ± 39.0 | 194.0 ± 35.7 | 0.771 |
| Triglycerides (mg/dl) | 268.4 ± 107.1* | 176.1 ± 87.3 | 147.3 ± 42.9 | 161.6 ± 70.1 | 0.001 |
| HDL-C (mg/dl) | 33.06 ± 13.4 | 37.61 ± 11.0† | 30.04 ± 8.5 | 40.81 ± 16.5 | 0.004 |
| LDL-C (mg/dl) | 114.4 ± 47.4‡ | 135.5 ± 32.6 | 150.4 ± 39.8 | 153.5 ± 38.3 | 0.027 |
| Vitamin C (ng/ml) | 0.86 ± 0.37 | 0.78 ± 0.29 | 0.95 ± 0.31 | 0.92 ± 0.34 | 0.358 |
| Calcium (mg/dl) | 9.56 ± 1.2 | 9.15 ± 1.0 | 9.33 ± 1.2 | 9.63 ± 1.0 | 0.498 |
| Parathyroid hormone (pg/ml) | 59.3 ± 10.6 | 52.6 ± 11.2 | 49.2 ± 12.3 | 54.7 ± 17.3 | 0.806 |
1Data are mean ± standard deviation (SD)
2Receiving 500 mg vitamin C per day
3Receiving 500 mg vitamin C per day plus 30 minutes endurance physical activity
4Receiving one placebo per day
5Receiving one placebo per day plus 30 minutes endurance physical activity
6Obtained from ANOVA or chi-square test, where appropriate
*P<0.05 compared with the other groups, using Tukey’s test
†P<0.05 compared with the placebo group, using Tukey’s test
‡P<0.05 compared with the placebo plus physical activity group, using Tukey’s test
PA: Physical activity
Table 1: Baseline characteristics of study participants.
On the other hand, the difference of the measured variables between the study groups after 12 weeks of intervention was shown in Table 2. As shown in the table we observed a significant increase in mean serum vitamin C concentrations in participants who received either vitamin C or “vitamin C plus 30 minutes/day of endurance PA” in compared with group took placebo or “placebo plus 30 minutes/day of endurance PA” (p value=0.001 for all). In addition, a significant decrease in mean serum calcium levels in participants who received either “vitamin C plus 30 minutes/day of endurance PA” or “placebo plus 30 minutes/day of endurance PA” in compared with group took placebo (p value <0.05 for all). Furthermore, a significant decrease in mean PTH levels in participants who received vitamin C or “vitamin C plus 30 minutes/day of endurance PA” in compared with group took placebo (p value<0.05 for all). Also, taking “vitamin C plus 30 minutes/day of endurance PA” lowered PTH more in compared with only taking vitamin C (-5.77 ± 7.29 vs. -4.98 ± 9.54). Moreover, the end of trial means of vitamin C, calcium, and PTH among study groups is shown in Table 3. After intervention, we observed a significant increase in mean serum vitamin C concentrations in participants who received vitamin C or “vitamin C plus 30 minutes/day of endurance PA” compared with other two groups (p value<0.001). Additionally, we observed a significant decrease in mean serum calcium concentrations in participants who received vitamin C or “vitamin C plus 30 minutes/day of endurance PA” and “placebo plus 30 minutes/day of endurance PA” (p value<0.001). Also, we observed a significant decrease in mean serum levels of PTH in participants who received vitamin C or “vitamin C plus 30 minutes/day of endurance PA” compared with other two groups (p value<0.001).
| Variables | Vitamin C2 (n=26) | p value6 |
Vitamin C+PA3 (n=23) | p value6 |
||
|---|---|---|---|---|---|---|
| Before Mean ± SD |
After Mean ± SD |
Before Mean ± SD |
After Mean ± SD |
|||
| Vit C (ng/ml) | 0.86 ± 0.37 | 1.36 ± 0.28 | 0.001 | 0.78 ± 0.29 | 1.66 ± 0.33 | 0.001 |
| Ca (mg/dl) | 9.56 ± 1.2 | 9.41 ± 0.8 | 0.319 | 9.15 ± 1.0 | 8.82 ± 1.6 | 0.028 |
| PTH (pg/ml) | 59.3 ± 10.6 | 54.3 ± 10.6 | 0.006 | 52.6 ± 11.2 | 46.83 ± 8.3 | 0.001 |
| Variables | Placebo4 (n=25) | p value6 |
Placebo+PA5 (n=22) | p value6 |
||
| Before Mean ± SD |
After Mean ± SD |
Before Mean ± SD |
After Mean ± SD |
|||
| Vit C (ng/ml) | 0.95 ± 0.31 | 0.88 ± 0.35 | 0.475 | 0.92 ± 0.34 | 1.07 ± 0.29 | 0.144 |
| Ca (mg/dl) | 9.33 ± 1.2 | 9.61 ± 1.0 | 0.041 | 9.63 ± 1.0 | 9.23 ± 0.5 | 0.015 |
| PTH (pg/ml) | 49.2 ± 12.3 | 54.61 ± 8.6 | 0.021 | 54.7 ± 17.3 | 52.8 ± 11.0 | 0.406 |
1Data are means ± standard deviation (SD)
2Receiving 500 mg vitamin C per day
3Receiving 500 mg vitamin C per day plus 30 minutes’ endurance physical activity
4Receiving one placebo per day
5Receiving one placebo per day plus 30 minutes endurance physical activity
6Obtained from paired sample T test
*P<0.05 are significant
PA: Physical activity; PTH: Parathyroid hormone; Vit C: Vitamin C; Ca: Calcium
Table 2: Difference of the measured variables between the groups at 12 weeks of intervention.
| Groups (96) | |||||
|---|---|---|---|---|---|
| Variables | Vitamin C2 (n=26) |
Vitamin C+PA3 (n=23) |
Placebo4 (n=25) |
Placebo+PA5 (n=22) |
p-value6 |
| Vitamin C (ng/ml) | 1.36 ± 0.28 | 1.66 ± 0.33 | 0.88 ± 0.35 | 1.07 ± 0.29 | 0.001 |
| Calcium (mg/dl) | 9.41 ± 0.8 | 8.82 ± 1.6 | 9.61 ± 1.0 | 9.23 ± 0.5 | 0.001 |
| PTH (pg/ml) | 54.32 ± 10.6 | 46.83 ± 8.3 | 54.61± 8.6 | 52.81 ± 11.0 | 0.001 |
1Data are means ± standard deviation (SD)
2Receiving 500 mg vitamin C per day
3Receiving 500 mg vitamin C per day plus 30 minutes endurance physical activity
4Receiving one placebo per day
5Receiving one placebo per day plus 30 minutes endurance physical activity
6Obtained from ANOVA
PA: Physical activity; PTH: Parathyroid hormone
Table 3: End of trial means of vitamin C, calcium, and parathyroid hormone across study groups.1
Finally, the changes in vitamin C, calcium, and PTH in different groups are presented in Table 4. Participants in the “vitamin C plus 30 minutes/day of endurance PA” group had greater changes in vitamin C compared with the other groups (p value<0.001). In addition, a significant change was observed and increased of serum calcium and PTH concentrations in placebo group compared to other groups (p value<0.001 for all). To summarize the findings, analyses showed some of the interventions were effective on decreasing PTH; some interventions increased vitamin C and furthermore decreased calcium levels in MetS patients.
| Variables | Vitamin C2 (n=26) |
Vitamin C+PA3 (n=23) |
Placebo4 (n=25) | Placebo+PA5 (n=22) |
p value6 |
|---|---|---|---|---|---|
| Vitamin C (ng/ml) | 0.49 ± 91 | 0.88 ± 04* | 0.06 ± 96 | 0.14 ± 95 | 0.001 |
| Calcium (mg/dl) | -0.15 ± 0.04 | -0.32 ± 0.94 | 0.27 ± 0.98* | -0.40 ± 0.05 | 0.001 |
| PTH (pg/ml) | -4.98 ± 9.54 | -5.77 ± 7.29 | 5.40 ± 0.63* | -1.90 ± 0.62 | 0.001 |
1Data are means ± standard deviation (SD)
2Receiving 500 mg vitamin C per day
3Receiving 500 mg vitamin C per day plus 30 minutes endurance physical activity
4Receiving one placebo per day
5Receiving one placebo per day plus 30 minutes endurance physical activity
6Obtained from ANOVA
*P<0.05 compared with the other groups
PA: Physical activity; PTH: Parathyroid hormone
Table 4: Changes in vitamin C, calcium, and parathyroid hormone in different groups.
To the best of our knowledge, this is the first intervention study, which investigated the effects of vitamin C supplementation along with and without endurance PA on calcium and PTH levels in MetS patients. The main findings of this study indicate that, daily supplementation of vitamin C (500 mg/ day), for 12 weeks along with moderate endurance PA had more effects than vitamin C alone, in order to normalize PTH and calcium levels in MetS patients. Various studies have shown the contradictory effect of vitamin C on PTH secretion, however some of the investigations showed its beneficial property to lower serum PTH level [22,23]. However, the interactive effect of vitamin C supplementation along with endurance PA on the keeping calcium and PTH levels to decrease the prevalence of MetS have not yet been concurrently estimated at the level of the general population. Richter and colleagues proposed that there was an inverse interaction between PTH level and vitamin C [22]. In addition, Sanadgol et al. [23] show that, increasing vitamin C levels by dietary supplementation results in a decrease of PTH in vitamin C deficient hemodialysis patients with secondary hyperparathyroidism. The results of our study support these findings. Vitamin C supplementation is possibly a modality to reduce PTH with less side effects, while several investigations have shown, increasing vitamin C levels by dietary supplementation resulted in reduction of PTH in vitamin C deficient individuals [24,25]. Furthermore, investigations have shown, in low serum levels of vitamin C, calcium-sensing receptors may become resistant to PTH effects and, vitamin C increases the response to PTH at the receptor level, through increasing the cyclic adenosine monophosphate and reducing PTH by it [32,33]. Moreover, our findings demonstrate that, after 12 weeks of intervention, taking vitamin C or “vitamin C plus 30 minutes/day of endurance PA”, lowered PTH more in compared with other two groups. Also, taking vitamin C plus 30 minutes/day of endurance PA, lowered PTH more in compared with only taking vitamin C. Evidence from other findings are in line with our finding shows that the synthesis and secretion of PTH increased in response to low vitamin C levels, in order to keep up calcium homeostasis [33]. Other shows no increase in PTH secretion [34], because of “functional hyperparathyroidism” [35] and normal kidney function [36] or may be because of decrease in plasma ionized calcium during PA [37]. Additionally, previous reports had shown that PTH may encourage calcium channel to increase calcium invasion, beside high calcium intake associated with lower PTH levels and decrease the risk of metabolic disease [38,39]. Furthermore, other factors rather than vitamin C such as vitamin D, age, gender, smoking status and serum calcium levels may contribute to PTH levels [40].
On the other hand, our findings reviled that, calcium levels were lowered in those who took vitamin C or “vitamin C plus 30 minutes/day endurance of PA” and “placebo plus 30 minutes/day of endurance PA”. The inverse relationship between calcium intake and plasma PTH levels has been widely studied at different ages and physiological stages in both humans and animals, in acute and long-term studies [41,42]. In the present study a decrease in calcemia values with the three types of intervention being more notorious for the exercise group than for the vitamin C supplementation group, suggesting an effect of exercise on calcemia, independent of vitamin C. Moreover, a complex relationship between calcium and PTH levels responses to PA have been observed previously [15,43]. Despite the beneficial effect of PA in treatment of low bone mineral density, high intensity PA are resulted in increased ionized calcium, no change in PTH levels, no longer have muscles mass and strength [44,45]. However, the disruption of calcium homeostasis is dependent on the duration of PA [46].
Finally, the findings of the present study shows that, MetS participants with vitamin C supplement plus 30 minutes/day of endurance PA were more PTH suppress in compared with both the placebo groups and those who only took vitamin C. In contrast to our finding, recent studies suggested that, higher levels of PTH were observed in higher physical active young person [42,47]. This change may be because of different in PA duration, age, gender, dietary habits, vitamin and mineral supplementation, as well as the characteristics of participants. Therefore, it need to be answer the hypothesis, if we increase the levels of vitamin C and normalize the PTH levels as well as retaining calcium balance, it is possible to prevent and decrease the prevalence of MetS. For all that, we are inattentive of former investigating in addition to vitamin C, hormones are also correlated with the MetS. Further studies are required.
The strength of the present study is that this is the first study, which investigated the effects of vitamin C supplementation along with and without endurance PA on calcium and PTH levels in MetS patients. Current study has adequate power to detect the significant effects of PA and vitamin C supplement in MetS patients; however, further studies with longer duration of interventions might be needed to confirm the long-term effects of PA and vitamin C supplementation in MetS patients.
In conclusion, adding PA to vitamin C supplement might be particularly important in regulation of PTH and serum calcium levels in MetS patients.
Ethical Issues
The Ethics Committee of Tehran University of Medical Sciences approved the study protocol, and the trial was registered at the World Health Organization, International Clinical Trails Registry Platform (Code: IRCT20161110030823N2). In addition, written informed consent was also obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not: for: profit sectors.
Authors’ contributions
AA, HAF, MT, RQ and AHB participated in the design of the study, data collection, performed the statistical analysis and drafted the manuscript. KD and AHB supervising the study and participated in draft review. All authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Acknowledgments
The authors wish to thank and appreciate the staff and participants at Halabja hospital, Kurdistan Region of Iraq for their important participation in the study.
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.