Research Article - (2021) Volume 10, Issue 10
An observational study was conducted in 201rheumatoid arthritis patients aged ≥ 18 years. Clinical response and DMARD use were monitored by laboratory parameters, objective signs and symptoms and assessment of patient health related quality of life questionnaire. The early use of DMARDs highlighted a decline in the severity of the disease. Treatment response was assessed by use of disease activity score of 28 joints (DAS28) using Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) and Short Form of Arthritis Impact Measurement Scale 2 (AIMS2SF). The commonly reported adverse effects included dyspepsia and hair loss which were manageable. The use of Combination DMARD therapy proved to be safe and efficacious in treating rheumatoid arthritis patients which are unaffected by non-steroidal anti-inflammatory drugs and analgesics.
Rheumatoid arthritis • Conventional disease modifying anti- rheumatic drugs • Disease activity score
Rheumatoid Arthritis (RA), a chronic inflammatory disease of joints, has a prevalence of 0.75% in India as of 2017 and varies widely in the Indian population. The treatment of RA aims to achieve the lowest possible level of disease activity and remission if possible, to minimize joint damage, and enhance physical function and quality of life. Untreated RA leads to several complications affecting various parts of the body, thus highlighting the importance of early treatment of RA with DMARDs [1]. The Rheumatoid Arthritis (RA) is associated with substantial long- term morbidity, mortality, and healthcare costs. Disease-Modifying Anti- Rheumatic Drugs (DMARDs) control disease activity, reduce joint erosions and improve quality of life as well as reduce cardiovascular morbidity associated with RA such as ischemic heart disease. In recent years, there has been a change towards the early and more dynamic treatment of RA. Early diagnosis of RA prompted the use of DMARDs in higher doses and often in combination therapy to control the disease activity.
However, population‐based analyses of DMARD use in patients with different indications are lacking, although its use has been extended. Therefore, the primary objective of this study was to analyze the population‐based frequency of c‐DMARD use in India. Also, we examined underlying indications and the specialty of the physicians prescribing DMARD in the study year available to us at the time of the analysis. The present study examined the efficacy and safety of DMARDs in RA patients.
An observational cross-sectional study was conducted in two medical centers, a tertiary care multi-specialty hospital, and a private rheumatology clinic, for six months. The study included RA patient’s ≥ 18 years that were on conventional DMARDs for more than 2 weeks. Patients with any major comorbidities like cardiovascular diseases, HIV-AIDS and cancers, pregnant or lactating women, and children, or immune compromised patients were excluded from the study. Written informed consent was obtained from each patient enrolled in the study on the prepared Patient Profile Form (PPF).
Questionnaires like the Arthritis Impact Measurement scale (AIMS2SF) were filled before enrollment and on follow-up visits. The Disease Activity Scoring (DAS 28) index was measured using the Erythrocyte Sedimentation Rate (ESR) or C-Reactive Protein (CRP) values. AIMS2SF scoring was done by first dividing the questions into five domains, each of which were scored and summed into scores ranging from 0-10, where higher scores indicated a higher severity of the disease.
The patients were screened for any Adverse Drug Reactions (ADRs). ADRs were assessed for severity and causality using the WHO-UMC Causality Assessment Scale and Hartwig’s Severity Assessment Scale.
Statistical analysis
All these results were calculated, and compared using statistical analyses, where the continuous variables were expressed as Mean ± SD. The differences in the means between the groups were assessed by the student ‘t’ test.
The percentages of all possible observations were calculated and compared between the groups by Chi-square test and p-value <0.05 was considered statistically significant.
In total, 201 patients were enrolled and assessed for conventional DMARD response to therapy, the pattern of adherence, and the safety profile of the drug (Table 1).
| Category | No. of subjects | |
|---|---|---|
| Age | No.% | Mean ± SD |
| 20-35 | 39 19.4% | 30 ± 4.74 |
| 36-50 | 78 38.8% | 42.95 ± 4.37 |
| 51-65 | 74 36.81% | 56.82 ± 3.74 |
| 66-80 | 11 5.41% | 71.91 ± 3.75 |
| Gender | ||
| Male | 19 | 9.45 |
| Female | 182 | 90.54 |
| Rheumatoid factor | ||
| Sero positive | 55 | 27.36 |
| Sero negative | 19 | 9.45 |
| NA | 127 | 63.18 |
Note: P-value<0.0001 for all the categories
Table 1. Demographic details of patients.
Statistical analyses showed that the patients in the age group of 36- 50 years were significantly higher as compared to those in the groups 20-35 (p<0.0001) and 66-80 (p<0.0001) while there was no significant difference between the population in the groups 36-50 and 51-65 (p>0.05). Female patients (90.54%) show statistically higher significant difference (p<0.0001) than male patients (9.45%).
The duration of disease condition annually was Mean ± SD of 5.65 ± 5.40 and in months had a Mean ± SD of 4.26 ± 2.47. Patients preferred primary care clinic (70.14%) over tertiary care (29.85%) for initial evaluation. The most observed signs and symptoms were multiple joint pain (52.73%), swollen joints (80.09%), tender joints (64.17%), and morning stiffness (50.24%). The least observed signs and symptoms include deformities (6.97%) (Table 2).
| DMARDS | No. of patients | %n=29 |
|---|---|---|
| HCQS | 12 | 41.38 |
| MTX | 8 | 27.59 |
| SSZ | 4 | 13.8 |
| LEFN | 5 | 17.2 |
| Combination therapy | No. of patients | % n=172 |
| DMARD combination | 156 | 68.02 |
| DMARD+Corticosteroids | 10 | 25.6 |
| Synthetic DMARDS+Biologics | 6 | 1.16 |
Note: DMARDs: Disease-Modifying Anti-Rheumatic Drugs
Table 2. The distribution of frequencies of monotherapy and combination approach for the latest treatment in patients with RA.
The prescription pattern revealed that DMARDs were given either as monotherapy (14.42%) or in combination (85.57%). hydroxychloroquine (41.38%) was the preferred choice of drug in monotherapy. A combination of two or more DMARDs (68.02%), methotrexate with leflunomide, or a triple regimen of methotrexate with hydroxychloroquine and sulfasalazine was the preferred combination therapy (Table 3).
| DAS (ESR and CRP) | No. of patients | % n=201 | No. of patients | % n=201 |
|---|---|---|---|---|
| High | 75 | 37.30% | 11 | 7% |
| Moderate | 106 | 52.50% | 40 | 19% |
| Low | 20 | 10.20% | 150 | 74% |
| ESR value (mean ± SD); Before treatment: 5.71 ± 0.88; After treatment: 4.95 ± 2.35; chai square value<0.0001 | ||||
| High | 69 | 34% | 25 | 12% |
| Moderate | 74 | 36% | 37 | 18% |
| Low | 58 | 30% | 139 | 69% |
Note: DAS: Disease Activity Scoring; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; ESR value (mean ± SD) before treatment: 5.69 ± 2.41; after treatment: 4.25 ± 2.34; chai-square value<0.0001
Table 3. Disease Activity Score 28, Erythrocyte Sedimentation Rate (DAS 28 ESR) and C-Reactive Protein (CRP) Data.
The data presented in Table 4 reveal that patients were adherent to the DAS 28 ESR and DAS 28 CRP test before and after treatment. Their results revealed a significant decrease in the disease activity post-therapy with DMARDs.
| Physical functioning | Symptoms | Affect | Social | |||||
|---|---|---|---|---|---|---|---|---|
| AIMS2SF | BT | AT | BT | AT | BT | AT | BT | AT |
| <3 Mild | 39 | 96 | 59 | 85 | 65 | 92 | 20 | 134 |
| -19.40% | -47.70% | -29.30% | -42.20% | -32.30% | -45.70% | -9.90% | -66.60% | |
| 3-5 Moderate | 132 | 78 | 102 | 98 | 120 | 70 | 141 | 47 |
| -65.60% | -38.80% | -50.70% | -48.70% | -59.70% | -34.80% | -70.10% | -23.30% | |
| >6 Severity | 30 | 27 | 40 | 18 | 16 | 39 | 40 | 20 |
| -14.90% | -13.40% | -19.90% | -8.90% | -7.90% | -19.40% | -19.90% | -9.90% | |
Table 4. Arthritis Impact Measurement scale (AIMS2SF).
The various parameters of AIMS2SF were assessed before enrolment and after treatment, which showed a significant (p<0.05) improvement in response to treatment and medication adherence. ¬WHO Causality Assessment Scale (Table 5) reveals that 41 patients encountered ADRs of which 73.17% were probable, 24.39% were possible and 2.43% were certain with a significant difference in comparing certain and probable cases (p<0.0001). According to Hartwig’s Severity Assessment Scale, the highest cases reported were level 1 and level 2 (mild symptoms) (Figure 1).
| Parameters | No. of cases | % n=41 |
|---|---|---|
| Certain (a) | 1 | 2.43 |
| Probable/Likely (b) | 30 | 73.17 |
| Possible (c) | 10 | 24.39 |
| Parameters | No. of cases | % n=41 |
| Level 1 (a) | 15 | 36.58 |
| Level 2 (b) | 17 | 41.46 |
| Level 3 (c) | 1 | 2.43 |
| Level 4 (d) | 6 | 14.63 |
| Level 5 (e) | 2 | 4.87 |
Table 5. Measure of the severity of DMARDs induced adverse drug reactions.
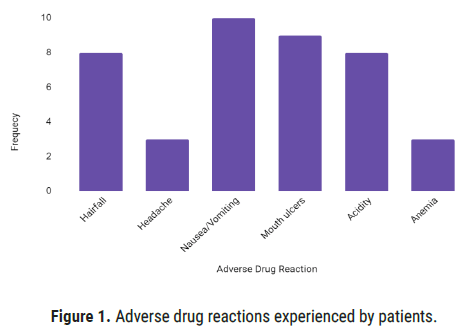
Figure 1: Adverse drug reactions experienced by patients.
The ADR’s occurred during the phase of the study was manageable and did not require cessation of drugs. The reactions were managed by lowering of dose of the drug and addition of supplemental drugs like biotin for hair fall, multivitamins for anemia, and antacids for acidity and ulcers. For few patients with severe ADR’s and increased disease activity, the patients were shifted to Biological DMARD such as Adalimumab and Rituximab.
In this study, RA was seen commonly in females with a male to female ratio of 1:9.6. The majority of patients were between 36 to 50 years of age. A recent study from a very large cross-sectional international cohort demonstrated slightly worse levels of disease activity and function in female patients with RA, compared with men. These findings are discussed in the context of our evolving knowledge of differences in the expression of this prototypic autoimmune disease, both in terms of the actual disease activity level, the effects that the disease has on physical function, and our ability accurately to measure these aspects.
The more prominent symptoms included morning stiffness (50.24%), tender joints (64.17%), and swollen joints (80.09%). About 49.75% of patients were found not to present an occurrence of morning stiffness which was consistent [2]. The degree of morning stiffness reflects functional disability and pain more than the presence of traditional markers of inflammation.
The numbers of prescriptions with DMARD monotherapy and combination therapy were 29 and 172 respectively. In monotherapy, hydroxychloroquine (41.38%) was the most commonly prescribed drug, followed by methotrexate (27.59%), sulfasalazine (13.8%), and leflunomide (10.34%). In combination therapy, the most prescribed patterns were two or more DMARD combinations (68.02%). Combination therapy with DMARDs is more effective than monotherapy in the treatment of rheumatoid arthritis. Folic acid was added to prevent methotrexateinduced anemia.
Assessment of Health-Related Quality of Life (HRQoL) was done by using Health Assessment Questionnaire (HAQ) viz. AIMS2SF. According to the AIMS2 is a shorter version of the AIMS and has been developed to assess the impact of arthritis on patient's quality of life. This short version is more concise and acceptable to patients than AIMS while it takes a shorter time to be completed [3]. Items tapping 5 core domains of the AIMS2SF were physical functioning, symptoms, social interaction, role, and affect. Scores for the different domains are summed and converted between ranges of 0-10.
The severity of ADRs was assessed using the Naranjo Causality Assessment Scale, WHO-UMC Causality Assessment Scale, and Hartwig’s Severity Assessment Scale which showed a higher number of probable and Level 2 cases, which were in line with studies conducted in Spain. ADR’s reported were hair loss, headache, nausea, mouth ulcers, vomiting, anemia, dyspepsia. Most of these ADRs reported were by the use of Methotrexate which can be co-related from a western study that described the adverse effects to DMARDs conducted by Carolina [4]. Although methotrexate is the widely used DMARD, there is a lack of evidence to predict with great accuracy who will respond well to treatment and who will develop adverse drug events but as there is decreased disease activity seen through DAS 28 in most patients. Low-dose methotrexate therapy is relatively well tolerated, provided that there is a careful patient selection and regular monitoring for adverse effects and drug interactions during methotrexate therapy is carried out [5]. The long-term clinical efficacy and relative safety of methotrexate remain impressive. Also, as most of the symptoms were managed by lowering of dose and changing the route of administration of drugs. The need omitting of methotrexate was not required but for some patients with severe ADR’s the drug was changed to other combinations mainly hydroxychloroquine and sulfasalazine which showed significant control on disease activity.
In this study, a combination of two or more DMARDs was efficacious in treating signs and symptoms of rheumatoid arthritis. DMARDs showed a considerable improvement in the disease activity and better adherence according to the DAS Scoring indices and AIMS2SF thereby improving patient’s health-related quality of life. The advances in rheumatoid arthritis therapy over the last 20 years have markedly changed the way the disease is managed and have improved outcomes. Understanding the therapeutic goals and the options available to achieve them, pre-treatment evaluation, and the ongoing monitoring for complications of the disease and its treatment will ensure the best outcomes for patients.
Citation: Raut A. “Therapeutic Response of Disease Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis”. J Arthritis, 2021, 10(10), 001-003
Received: 02-Sep-2021 Published: 23-Sep-2021
Copyright: © 2021 Raut A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.