Short Communication - (2017) Volume 6, Issue 1
Inflammatory response and calcification are strongly implicated in osteoarthritis (OA) progression. Key inflammatory biomarkers present throughout the process of OA have been established but an association with calcification has not been clarified. A faint line, tidemark, exists between the subchondral bone and the articular surface in knee joints that is presumed to be the line of demarcation between calcified and uncalcified cartilage. This study shows that the tidemark is where calcification is actively occurring. Mouse knees (n=8) were stained for both Alkaline Phosphatase (ALP) and Safranin-O/Fast Green. ALP, active during tissue calcification, was used to identify the true location of calcification. Images from both stains were overlaid for comparison of complementary tissues. The cartilage area above the tidemark was then compared to the area above the ALP line of calcification. Because the two areas proved to be nearly statistically identical, the conclusion is that the tidemark observed in the Safranin- O/Fast Green stain is indeed the line of calcification for articular cartilage. The role of calcification in OA was further examined by comparing NPP1, calcification marker, with HtrA1. We demonstrate a reciprocal correlation between HtrA1 and NPP1, suggesting a link between pathological calcification and inflammation in joints experiencing OA.
Keywords: Osteoarthritis; Tidemark; Calcification; NPP1; HtrA1; ALP
Osteoarthritis (OA) is one of the most common chronic diseases in the United States and its prevalence is increasing due to the aging and obesity of the population. OA is characterized by joint pain and effusion, loss of mobility and deformity that can progress to functional joint failure. More than 80% of people older than 65 years are symptomatic for OA [1-3]. Conservative estimates indicate that patients with OA in the United States account for 46 million physician visits with an estimated total cost of more than $115 billion for the years 1989 through 1991 [4-6].
Formerly, OA was thought to be a disease of ‘wear and tear’, a disorder in which mechanical forces physically degrade the articular cartilage (AC). Although avascular, articular cartilage is now recognized to be metabolically active and OA is viewed as a dynamic process triggered by a variety of mechanisms [7-10].
Articular cartilage is made up of two layers mainly distinguished by the presence or absence of calcification. The deeper layer of hyaline articular cartilage (HAC) becomes articular calcified cartilage (ACC) [11-16]. Mineralization of articular cartilage to form HAC results in a loss of pliability and compression but allows for resorption by osteoclasts in the process of bone formation on the eroded surface. Thus cartilage is contiguous with bone instead of just an appendage attached to it. This feature appears to be critical to the function of joints. The ultrastructural nature of deep HAC and all ACC is associated with and a function of the surface orientation of the cartilage collagen. In contrast, the subchondral bone collagen is distinguished as a layered mat in which fibers are parallel to the resorption interface. In this way subchondral bone collagen is better designed to prevent cleaving and/or cracking.
Histological stains of osteoarthritic murine knee joints for proteoglycans, such as Safranin-O, reveal a faint line of demarcation between calcified and uncalcified cartilage known as a tidemark [17-20]. This observation suggests that the cartilage becomes metabolically calcified in an induced conversion of HAC into ACC, during the onset and progression of OA [11,21-23].
It has also been shown that inflammation can be related to the onset of OA [24-26]. Inflammation is associated with the upregulation of HtrA1 which degrades aggrecan and other proteoglycans in the pericellular matrix (PCM) [27]. This causes the density of the PCM to change such that Type II collagen from the extracellular matrix (ECM) is absorbed into the PCM. Polur et al. have shown that the presence of HtrA1 facilitates Type II Collagen binding to the Ddr2 receptor which, through intracellular signaling, upregulates the expression of Mmp-13 [28].
Mmp-13 is a matrix metalloproteinase that digests Type II collagen and ultimately contributes to the mechanical destabilization of the ECM [29]. Mmp-13 has also been associated with inflammation triggered OA [26,30].
Ectonucleotide pyrophosphatase/phosphodiesterase-1 (NPP1) has been shown to create extracellular pyrophosphate (PPi) and prevent pathological calcification of cartilage and soft tissue [31]. Inborn NPP1 deficiency is associated with pathological calcification of arteries in mice and humans [32,33].
We hypothesize that relative levels of NPP1 decrease as expression of the inflammatory biomarker HtrA1 increases early in osteoarthritic mice. This would suggest that osteoarthritic calcification and
inflammation are correlated. To test this hypothesis destabilization of the medial meniscus (DMM) surgery was performed on mice (n=5) to induce OA. Knees were harvested 28 days post-surgery along with control/non-surgery mice. Tissue samples were stained for NPP1 and HtrA1 in both the control group and the 28 days post-DMM surgery group. The number of cells staining positive for HtrA1 and NPP1 were counted to test for an inverse correlation of these biomarkers between the control and surgery groups.
Mice
Normal, wild-type (wt) mice were euthanized by carbon dioxide at ages less than 16 weeks (n=5) and older than 32 weeks (n=3) in order to evaluate knee joint biology over a lengthy time course. The two different age groups were used to help prove that the correlation of the tidemark location between the stains is independent of specimen age. The handling of mice specimens was approved by the Brigham Young University Institutional Animal Care and Use Committee (IACUC) and assigned protocol #13-0601. Brigham Young University has an assurance document from the Federal Office of Laboratory Animal Welfare that authorizes it to use animals in scientific research as overseen by the IACUC.
Tissue processing
Right knee joints of mice were excised and fixed in periodate-lysineparaformaldehyde (PLP) fixative (2% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate solution, pH 7.4) for 24 hours at 4°C [34]. Tissues were then placed for 12 hours in a series of phosphate-buffered saline (PBS) washes containing varying amounts of glycerol according to previously published procedures [34]. Following fixation, tissues were decalcified in a series of Ethylene diamine tetra acetic acid (EDTA) solutions for 10-14 days. Then another series of PBS washes were followed to remove EDTA and glycerol from the samples. Tissues were then processed and embedded in paraffin. For a detailed a list of procedures and solutions, see Miao and Scutt, 2002 [34]. Tissue blocks were sectioned at 6 μm sagittal cuts at the front of the tibiae using a microtome. Three to five consecutive slices were placed in a warm bath at 40°C and then applied to the hydrophobic side of microscopic slides. These slides were then set on heating pad to dry.
Histological analysis
ALP Staining: Tissues on slides were deparaffinized using graded series of xylene, alcohol, and PBS washes. After tissues were hydrated, they were preincubated overnight in 1% magnesium chloride in Trismaleate buffer (pH 9.2) at room temperature [34]. Tissues were then incubated for 2 hours at room temperature with ALP substrate solution (freshly prepared 100 mM Tris-maleate buffer, pH 9.2, containing 0.2 mg/ml naphthol AS-MX phosphate and 0.4 mg/ml Fast Red TR) [34]. Vector methyl green nuclear counterstain (Vector Laboratories; Peterborough, UK) was then applied. Tissues were then mounted with coverslips using Kaiser’s glycerol jelly and allowed to dry at room temperature. For a detailed list of the procedure of the ALP staining process see, Miao and Scutt, 2002 [34].
Safranin-O/Fast Green staining: Nearly identical tissue sections to those used in the ALP stain were stained using Safranin-O/Fast Green. To do this, sequential sections from either before or after an ALP stained slide were taken and stained. This was done in order to provide comparison of the two stains with little to no deviation in the anatomy of the tissue. As part of the Safranin-O/Fast Green stain, tissues were deparaffinized using a series of xylenes and alcohol washes. Tissues were then subjected to hematoxylin, Scotts buffer, fast green, acetic acid, Safranin-Orange, and series of alcohol and xylene washes. After the staining procedure, tissues were mounted with a coverslip using Cytoseal 280 (Richard-Allan Scientific® Histology/Cytology Reagents, Kalamazoo, MI, USA).
Adobe Photoshop Analysis: To compare accuracy of the ALP stain with the tidemark observed in the Safranin-O/Fast Green stain, cartilage measurements above the apparent tidemarks for each mouse specimen was captured using the Lasso Tool in Adobe Photoshop. This was done for each of the stains to measure the presumed area between the apical surface of the cartilage and where the articular cartilage presumably interfaces with previously ossified cartilage (ALP line in ALP stain or tidemark line in Safranin-O/Fast Green stain). The technique was perfected in our laboratory and reported at the 2011 Conference of the International Society of Osteoarthritis Research (OARSI) – see Osteoarthritis and Cartilage volume 19 (Supplement 1) S108. To help standardize the human measurement error, two different lab members independently measured the tissue samples. As mentioned above, knee samples were carefully sectioned and sequential slides were taken and used in separate stains to ensure nearly identical anatomy between compared ALP and Safranin-O/Fast Green areas.
Statistical analysis: Both the ALP and Safranin-O/Fast Green stains were independently measured twice by different lab members. A comparison of the inter-rater reliability of measurements both within the same stain and between the two stains was performed using the VARCOMP Procedure from the Statistical Analysis System (SAS) software. From this procedure the inter-rater reliability was computed from the variance estimates.
Mechanical induction of OA to study biomarkers
Surgery: To induce OA, destabilization of the medial meniscus (DMM) surgery was performed on physiologically normal, wt mice (n=5). Surgeries were performed on the right knee of each animal at 35 days of age. At 28 days post-surgery, the mice were euthanized by isoflurane USP (Abbott Laboratories, North Chicago, IL, USA). Control mice (n=5) were euthanized at the same age as the surgery group (2.25 months of age + 3 days). All procedures involving mice were conducted according to an animal use protocol previously approved by the Brigham Young University IACUC and was designated as protocol #13-0601 by the University IACUC.
Immunohistochemical analysis: Tissues on slides were deparaffinized and blocked for an hour with 5% bovine serum albumin and sodium azide [26]. NPP1 specific goat primary antibody (Abcam Cambridge, MA, USA ab40003) was then added to the tissue, and on separate, complementary slides HtrA1 primary rabbit antibody (Abcam Cambridge, MA, USA ab386) [35] was applied. Both NPP1 and HtrA1 antibodies were diluted 1:200 [9]. After addition of the antibodies, tissues were then left to incubate overnight at 4°C. The following day tissues were blocked by application of ABC mix and the secondary antibody was applied and peroxidase substrate was used as substrate. Tissues were then washed and mounted. For a detailed list of the procedure for the NPP1/HtrA1 staining process see, Larkin et al, 2013 [26].
ImageJ Analysis: The expression levels of NPP1 and HtrA1 were analyzed quantitatively using the Java based imaging processing program, ImageJ (National Institutes of Health, Bethesda, MD, USA). In this analysis, cells that stained positive for either NPP1 or HtrA1 were counted in defined pixel areas of cartilage [26]. The percentage of cells per defined area was then statistically analyzed for significance.
A reliability measurement between the Safranin-O and ALP stains of 0.991 was achieved. This means there was a 99.1% chance that the stains were demarcating the same area as the hypothesized tidemark.
A reliability of 0.987 was measured between the measurements done by different lab members within each of the stains (Figure 1). This reliability shows that our method of measurement was accurate independent of the person using the Lasso Tool in Photoshop.
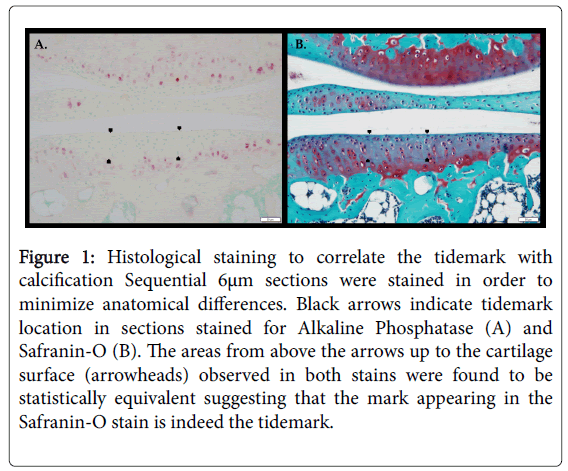
Figure 1: Histological staining to correlate the tidemark with calcification Sequential 6μm sections were stained in order to minimize anatomical differences. Black arrows indicate tidemark location in sections stained for Alkaline Phosphatase (A) and Safranin-O (B). The areas from above the arrows up to the cartilage surface (arrowheads) observed in both stains were found to be statistically equivalent suggesting that the mark appearing in the Safranin-O stain is indeed the tidemark.
An inverse relationship between HtrA1 and NPP1 expression when comparing the DMM surgery group to the age matched control group (Figure 2) was observed. The mean number of chondrocytes staining positive were statistically different for both HtrA1 (p<0.05) and NPP1 (p<0.01) (Figure 3) between control and age matched DMM surgery groups. The areas from above the arrows up to the cartilage surface (arrowheads) observed in both stains were found to be statistically equivalent suggesting that the mark appearing in the Safranin-O stain is indeed the tidemark.
Figure 2: Histological comparison of NPP1 and HtrA1 in age matched mice before and 28 days post DMM or control. Age matched control (A & B; n=5) and 28 days post DMM surgery (C & D; n=5) mouse knee sections (n=5 each group) were examined for the expression of NPP1 and HtrA1. As the disease progresses, a decrease in cells presenting NPP1 is observed 28 days after DMM surgery (A & C). Conversely, there is an increase in cells presenting HtrA1 (B & D) as the disease progresses after surgery.
Figure 3: NPP1 and HtrA1 detection in surgery and control mice. Using Image-J the percentage of cells staining positively for both NPP1 and HtrA1 were counted in knee sections from DMM (n=5) and control mice (n=5). Error bars were calculated with standard error. Comparing control to DMM surgery knees, there was a significant difference between the percentage of cells expressing NPP1* (p<0.01) and HtrA1** (p<0.05).
The work presented in this paper correlates actual calcification with OA and is important for several reasons. Fist, calcium deposition has been shown to play a significant role in OA pathology, including the articular cartilage [36-38]. Calcium deposition has been shown to lesson actual mechanical efficacy of the articular cartilage and associated menisci [38]. Indeed, presence of osteophytes are generally indicative of OA pathogenesis [39].
Second, previous work regarding scoring joints for OA severity have suggested that cellularity and tidemark cause the most scoring discrepancy and need more consensus and/or better illustrations [40]. Our study provides a more clear illustration of the correlation of the tidemark and calcification under a variety of OA severities. Third, related to point two, for the purposes of scoring OA damage, tidemark is defined as zone of increased calcification at the border of uncalcified and calcified cartilage. Our study confirms that assertion. Last, structural features associated with the bone/cartilage interface including tidemark advancement, tidemark duplication are important to scoring OA severity accurately [41]. Our study confirms that the tidemark is indeed a dynamic feature.
This work was born out of a previous study from our lab that called into question the likelihood that the line of calcification, i.e. tidemark, in murine knee joints was actually detected with a Safranin-O stain [29], we devised this study. We set out to determine if the delineating tidemark is in fact an area of active metabolic calcification and whether it progresses toward the apical surface of the cartilage as OA progresses. A histological stain was used for Alkaline Phosphatase (ALP), an enzyme involved in endochondral ossification and a known way of identifying regions of active calcification [42], to determine whether the faint line seen in Safranin-O staining was indeed the tidemark or area of calcification.
The tidemark observed in the Safranin-O stains aligns with the location of ALP activity, suggesting that it is indeed an area of metabolic calcification in the articular cartilage (Figure 1). After making this observation, attempts to determine whether or not this tidemark remains localized to the same area or if it progresses toward the apical surface with age were unsuccessful. This was due to morphological differences between the mouse samples and the uneven distribution of the tidemark, we were unable to confidently make any tidemark measurements across different age groups. This made it difficult to map changes in tidemark thickness across samples representing different stages of disease progression. However, based on the samples obtained, we speculate that the tidemark remains in the same location and does not progress toward the apical surface of the cartilage with age. While no statistically rigorous data were obtained to prove this, despite the uneven nature of the tidemark along the subchondral bone from sample to sample, we observed a fairly constant distance from the subchondral bone. This led to a reevaluation of our initial hypothesis that the tidemark moves upward with increasing severity of OA.
The surgical group had minimal staining for NPP1 compared to the control group. The surgical group had significantly more staining for HtrA1 compared to the control group. At the present time, it cannot be proved whether or not there is an inversely causational relationship between NPP1 levels and inflammation in the cartilage or whether they are simply inversely correlated. This is an area of further study that our lab will pursue in the future. According to data from Alkaline Phosphatase staining, the line of demarcation appearing in Safranin-O stained slides is, in fact, the location of cartilage undergoing calcification. This work demonstrates that there is a significant inverse correlation between inflammation and NPP1 expression. It can be concluded that metabolic calcification increases as inflammation increases. Whether or not these inflammatory markers directly stimulate calcification by inhibiting NPP1 expression remains to be seen.
The authors declare that there is no conflict of interest regarding the publication of this paper, either for commercial or financial, and any other personal gain.
Funding for this project was supplied in part by a grant from the Brigham Young University Office of Research and Creative Activities and a generous gift from Allen C. and Kathy Christensen.