Research Article - (2021) Volume 7, Issue 1
Introduction: Allergen immunotherapy (AIT) is a commonly treatment that decreases symptoms for people with allergic rhinitis (AR) and asthma (AA). Despite the wide use of AIT, the economic evaluation of AIT versus symptomatic treatment has not been well established.
Objective: To conduct a cost-effectiveness (CE) analysis of subcutaneous immunotherapy (SCIT) versus symptomatic treatment for AR and AA, in the Greek health care setting. The consequences of treatments were evaluated from a 3rdparty payer perspective in a 10-year time horizon.
Method: A Decision tree model was used to reflect the natural progression and evaluate the CE of the comparators. Efficacy and safety data considered in the model were extracted from literature review and published studies. Utilities values were extracted from the literature. Direct costs were incorporated in the model reflecting the year 2018. Probabilistic sensitivity analysis was conducted to account for uncertainty and variation in the parameters of the model.
Results: Discounted survival and quality adjusted survival of SCIT treated patients was higher compared to symptomatic treatment by 1.51 lifeyears and 0.89 QALYs. SCIT was more costly in terms of drug acquisition medications, but the total cost per patient was less costly for SCIT, due to lower cost of management of AR and AA. The total cost per patient was estimated at 7,522€, for SCIT and for symptomatic treatment at 10,230€. Probabilistic analysis confirmed the deterministic results.
Conclusion: Results suggest that SCIT may be a dominant alternative relative to symptomatic treatment in the treatment of patients with AR and AA.
Allergic rhinitis, Allergic asthma, Immunotherapy, Costeffectiveness, Symptomatic treatment
Allergic rhinitis (AR), also known as hay fever, is an IgE inflammatory response in the nasal mucosa following exposure to allergens that the subject is sensitized to [1]. Symptoms mainly include a rhino rhea and/or nasal congestion sneezing, pruritus as well as eye signs such as redness and itching/watery eyes [2]. Besides physical discomfort, AR patients often present sleep disturbances, school absenteeism, impaired work ability and quality of life [3]. Symptoms can occur either perennially, in the case of sensitization to house dust mites, animal dander, molds and cockroaches or triggered by seasonal allergens such as tree, weed and grass pollens.
AR affects up to 40% of the population worldwide. High prevalence is being recorded in the developed countries of the Northern Hemisphere, with 23-30% of the affected population being in Europe and 12-30% in the USA. In respect to the non-Western populations of the Southern Hemisphere, prevalence diversity is noted, with wide inter- and intra-regional variations, ranging from 2.9% to 54.1% between countries. The prevalence of seasonal AR is higher in children and adolescents, while perennial AR seems to be more common at a later age [4-13].
The inter-relation between AR and allergic asthma (AA) is an important factor when evaluating treatments for AR. A vast body of evidence supports that AR precedes the AA development, while others have shown that at least 80% of patients with AA also have AR [14,15].
According to international guidelines, treatment of AR includes allergen avoidance when possible [16]; pharmacotherapy, mainly with antihistamines, and/or nasal corticosteroids [16] and allergen immunotherapy (AIT)
Allergen Immunotherapy (AIT) imposes additional costs to third party payers because of long-term clinical superiority over symptomatic treatment. Cost-effectiveness (CEA) and cost-utility analyses (CUA) represent robust methodologies in order to quantify the benefits and to evaluate the trade-offs amongst available treatments. These types of analyses aim to determine treatments that generate increased value over the money spent per patient.
Hence, the objective of the present analysis was to assess and evaluate the cost-effectiveness of subcutaneous immunotherapy (SCIT) versus conventional treatment for AR and AA, in the Greek health care setting. Sublingual immunotherapy (SLIT) was not selected due to paucity of local data.
A Decision tree model was developed to reflect the natural progression of patients with AR and AA. The model evaluated the cost-effectiveness of SCIT versus symptomatic treatment, over a ten year time horizon in the course of a 1-month cycle (Figure 1). The analysis was performed from a payer’s perspective (EOPYY) in Greece. Costs and outcomes beyond the one year time-frame were discounted at a 3.5% annual rate which is the standard practice in Greece as well as other jurisdictions (Figure 1).
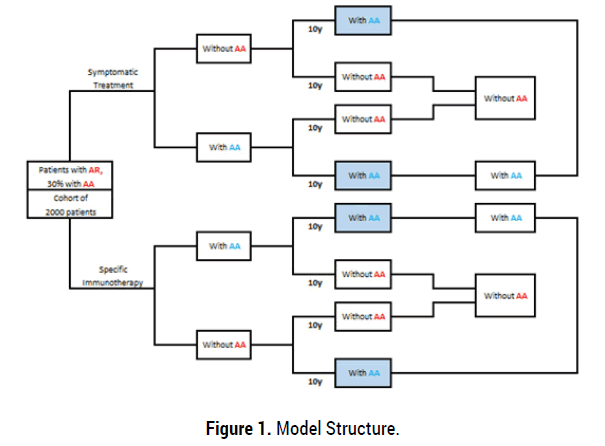
Figure 1. Model Structure.
Modeling Approach
The outcomes associated with each treatment option are estimated by means of a state transition Markov model, which was built to simulate over time the health status progression and the management of a patient cohort under the two hypothetical therapy scenarios. The model estimates in each case the mean expected survival, quality of life, health events, health care resource use and treatment cost. The simulation runs on a monthly cycle basis in ten year time horizon. Additional symptomatic treatment was allowed in the SCIT arm. SCIT duration was estimated at 3 years. No half cycle correction was necessary.
The model structure is based on predefined disease stages and corresponding transition probabilities for all treatment alternatives in order to predict the long-term course of disease in a specific patient cohort (Treatment alternatives included: SCIT and symptomatic treatment alone).
Based on the aforementioned, the following disease states were classified [9,18]: [i] mild allergic rhinitis, [ii] moderate to severe allergic rhinitis , [iii] moderate to severe allergic rhinitis and mild allergic asthma, [iv] moderate to severe allergic rhinitis and moderate to severe allergic asthma, [v] no symptoms, and [vi] dead. The transition probabilities between the predefined states were obtained directly or from published literature sources [19-24].
At the start of the model calculation, patients with AR had a mean age of 30 years, while a proportion of them (30%) presented concomitant AA.
During the model duration (as shown in Figure 1), AR patients were grouped in the presence of atopy, as having moderate or severe rhinitis (with or without asthma) or mild to severe asthma associated with AR. In the SCIT arm, 3 health states are considered: the patient becomes asymptomatic or improves (and continues to receive SCIT for 3 years) or following 2 pollen seasons the patient’s condition remains either unchanged nor aggravates and SCIT is discontinued while maintaining standard therapy. After 3 years of SCIT, 2 possibilities are considered: stabilization or worsening.
In the no SCIT arm, 3 possibilities are considered: improvement, stabilization, or aggravation; in the meantime of the analysis the patient continues convectional drug treatments.
The percentage of patients discontinuing SCIT prematurely was determined based on most recent European studies covering SCIT real-life observations on discontinuation events. Discontinuing patients were not allowed to re-initiate SCIT following discontinuation.
Model Assumptions
The model is based on the following assumptions: (1) the efficacy of SCIT is superior to current symptomatic treatment; (2) SCIT improves the natural history of AR; (3) following 3 years of SCIT a long-term efficacy is observed; (4) patients discontinuing SCIT after 2 pollen seasons, drugs have the same efficacy as in the symptomatic treatment arm; and (5) asymptomatic patients discontinue SCIT and drug therapy [25].
Clinical Inputs
The prevalence of patients in the categories “with asthma symptoms” and “without asthma symptoms” was estimated in 2 steps because of different disease courses. During the first step, estimates were based on each subpopulation at the start of treatment, including patients with asthma and patients without asthma symptoms, according to the parameter values given in Table 1 [24-51] (Tables 1-3).
| Parameters | Mean % (CI) |
|---|---|
| Prevalence of patients with asthma at the start of treatment [26-39] | 30 (9-44) |
| Cumulative incidence rate of asthma per year | |
| SCIT [40, 41] | 0.74 (0.46–1.02) |
| ST [31, 40, 42] | 2.52 ( 1.90–3.65) |
| Cumulative remission rate of asthma per year | |
| SCIT [41, 43] | 6.41 (4.35–7.64) |
| ST (natural remission) [44] | 2.45 |
| Excess mortality of patients with asthma per year [44-46] | 0.48 (0.25–0.70) |
| Reduction in the need for anti-allergic pharmacotherapy with SCIT [40, 42, 47-49] |
44% (21%–53%) |
| Reduction in the need for anti-allergic pharmacotherapy with ST [42, 50, 51] |
1.93% (1.26%–4.00%) |
SCIT: Subcutaneous Immunotherapy; ST: Symptomatic Treatment
Table 1. Clinical parameters.
| Incremental analysis | |||
|---|---|---|---|
| Outcomes | SCIT | ST | SCIT vs ST |
| Immunotherapy Cost | 2,091 € | - | 2,091 € |
| Allergic Rhinitis Cost | 3,403 € | 5,633 € | -2,230 € |
| Allergic Asthma Cost | 2,028 € | 4,597 € | -2,569 € |
| Total costs* | 7,522 € | 10,230 € | -2,708 € |
| Total QALYS* | 6.89 | 6.00 | 0.89 |
| Total Life Years* | 9.77 | 8.26 | 1.51 |
| Incremental cost per QALY | Dominant | ||
| Incremental cost per LY | Dominant |
SCIT: Subcutaneous Immunotherapy; ST: Symptomatic Treatment; QALYs: Quality Adjusted Life Years; LYs: Life Years; AR: Allergic Rhinitis; AA: Allergic Asthma
Table 2. Base case results for SCIT vs symptomatic treatmentfor the treatment of patients with AR and AA.
| Base case ICER | Dominant | Low value | High Value | ||
|---|---|---|---|---|---|
| Parameter | Base case value | High value | ICER | Low value | ICER |
| Prevalence of pts with asthma at the start of treatment | 0.30 | 0.33 | -3,264 € (Dominant) | 0.27 | -3,264 € (Dominant) |
| SCIT: Incidence rate of asthma per year | 0.01 | 0.01 | -3,244 € (Dominant) | 0.01 | -3,283 € (Dominant) |
| ST: Incidence rate of asthma per year | 0.03 | 0.03 | -3,378 € (Dominant) | 0.02 | -3,149 € (Dominant) |
| SCIT: Remission rate of asthma per year | 0.06 | 0.07 | -3,307 € (Dominant) | 0.06 | -3,219 € (Dominant) |
| ST: Remission rate of asthma per year | 0.02 | 0.03 | -3,222 € (Dominant) | 0.02 | -3,306 € (Dominant) |
| Excess Mortality of pts with asthma per year | 0.00 | 0.01 | -3,257 € (Dominant) | 0.00 | -3,270 € (Dominant) |
| Reduction for Therapy due to SCIT Year 1 | 0.91 | 1.00 | -3,207 € (Dominant) | 0.82 | -3,321 € (Dominant) |
| Reduction for Therapy due to SCIT Year 2 | 0.60 | 0.66 | -3,227 € (Dominant) | 0.54 | -3,300 € (Dominant) |
| Reduction for Therapy due to SCIT Year 3 | 0.44 | 0.48 | -2,918€ (Dominant) | 0.40 | -3,610 € (Dominant) |
| Reduction for Therapy due to ST Year 1 | 0.97 | 1.07 | -3,326 € (Dominant) | 0.87 | -3,201 € (Dominant) |
| Reduction for Therapy due to ST Year 2 | 0.75 | 0.83 | -3,312 € (Dominant) | 0.68 | -3,215 € (Dominant) |
| Reduction for Therapy due to ST Year 3 | 0.94 | 1.03 | -4,142€ (Dominant) | 0.85 | -2,385 € (Dominant) |
| Utilities: Mild Allergic Rhinitis | 0.76 | 0.83 | -3,065 € (Dominant) | 0.68 | -3,378 € (Dominant) |
| Utilities: Mod. to Sev. AR | 0.74 | 0.81 | -3,040 € (Dominant) | 0.66 | -3,521 € (Dominant) |
| Utilities: Mod. to Sev. AR and Mild Asthma | 0.73 | 0.80 | -3,162 € (Dominant) | 0.66 | -3,458 € (Dominant) |
| Utilities: Mod. to Sev. AR and Mod. to Sev. AA | 0.70 | 0.77 | -3,171 € (Dominant) | 0.63 | -3,362 € (Dominant) |
| Utilities: No Symptom | 0.78 | 0.86 | -3,289 € (Dominant) | 0.71 | -3,301 € (Dominant) |
| Death | 0.00 | 0.00 | -3,264 € (Dominant) | 0.00 | -3,264 € (Dominant) |
| Discounting: Effectiveness | 0.04 | 0.04 | -3,001 € (Dominant) | 0.03 | -3,584 € (Dominant) |
| Discounting: Cost | 0.04 | 0.04 | -3,264 € (Dominant) | 0.03 | -3,264€ (Dominant) |
| AR: Pharmaceutical Cost | 435.60 | 479.16 | -3,444 € (Dominant) | 392.04 | -3,083 € (Dominant) |
| AR: Medical Cost | 36.80 | 40.48 | -3,279 € (Dominant) | 33.12 | -3,248 € (Dominant) |
| AR: Hospitalization Cost | 80.00 | 88.00 | -3,297 € (Dominant) | 72.00 | -3,230 € (Dominant) |
| AR: ICU Cost | 2.00 | 2.20 | -3,264 € (Dominant) | 1.80 | -3,263 € (Dominant) |
| AR: Lab Tests Cost | 41.20 | 45.32 | -3,281 € (Dominant) | 37.08 | -3,247 € (Dominant) |
| AR: Functional/Imaging Tests Cost | 34.00 | 37.40 | -3,278 € (Dominant) | 30.60 | -3,249 € (Dominant) |
| AR: Additional resources cost | 0.00 | 0.00 | -3,264 € (Dominant) | 0.00 | -3,264 € (Dominant) |
| AA: Pharmaceutical Cost | 939.80 | 1033.78 | -3,452 € (Dominant) | 845.82 | -3,075 € (Dominant) |
| AA: Medical Cost | 75.40 | 82.94 | -3,279 € (Dominant) | 67.86 | -3,248 € (Dominant) |
| AA: Hospitalization Cost | 128.30 | 141.13 | -3,289 € (Dominant) | 115.47 | -3,238 € (Dominant) |
| AA: ICU Cost | 60.00 | 66.00 | -3,276 € (Dominant) | 54.00 | -3,252 € (Dominant) |
| AA: Lab Tests Cost | 100.00 | 110.00 | -3,284 € (Dominant) | 90.00 | -3,243 € (Dominant) |
| AA: Functional/Imaging Tests Cost | 69.70 | 76.67 | -3,278 € (Dominant) | 62.73 | -3,250 € (Dominant) |
| AA: Additional resources cost | 96.60 | 106.26 | -3,283 € (Dominant) | 86.94 | -3,244 € (Dominant) |
| Immunotherapy cost | 58.50 | 64.35 | -3,034 € (Dominant) | 52.65 | -3,493 € (Dominant) |
ICER: Incremental Cost – Effectiveness Ratio; SCIT: Subcutaneous Immunotherapy; ST: Symptomatic Treatment; QALYs: Quality Adjusted Life Years; LYs: Life Years; AR: Allergic Rhinitis; AA: Allergic Asthma
Table 3. Results from the sensitivity analysis.
In the initial subpopulation (patients with asthma), the decreasing prevalence of patients with asthma was given by [24]
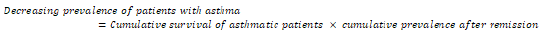
The increasing prevalence of patients without asthmatic symptoms was given by [24]

In the initial subpopulation (patients without asthma symptoms), the increasing prevalence of patients with asthma was given by [24]:
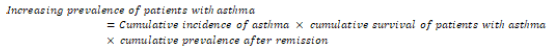
The decreasing prevalence of patients without asthma symptoms was given by [24]
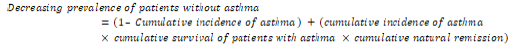
Reduction in the Need for Anti-Allergic Pharmacotherapy
The reduction in the need for anti-allergic pharmacotherapy (for AR and AA) after SCIT was derived from systematic reviews, showingthat SCIT is highly effective in AR, in patients with seasonal pollinosis[52] and also in patients with perennial allergy and sensitization to house dust mites [53]. Clinical efficacy is characterized by a marked reduction in requirements for AR medication during the pollen season. A randomized controlled trial of 410 patients with grass pollen allergy showed a 30% decrease in seasonal symptoms, a 44% reduction in need for anti-allergic medication and a marked improvement in quality of life during the pollen season [54]. AIT is also effective in AA. A Cochrane review [55,56] demonstrated significant improvements in symptoms, reduction in rescue medication, and improvements in allergenspecific bronchial hyper responsiveness. Immunotherapy was particularly effective in seasonal asthma [57].
Over time reduction in the need for anti-allergic pharmacotherapy after SIT were estimated based on data corresponding to the population considered in the further analysis of Pokladnikova et al. [58], which reflects the population of this study [59]. Follow up data of reduction in the need for anti-allergic pharmacotherapy after SIT were available in 1st, 2nd and 3rd year were used to estimate rates and linear interpolation was used to get estimates for the intermediate months. The last observation in 3rd year is carried forward.
Annual reduction rates derived from the analysis are converted to monthly ones using the following formula:
Reduction rate =1-(1-annual reduction rate) ^ (1/12).
Utilities
Utilities in the Markov model were applied by health state and not by treatment arm. The utility values were derived from the study of Bernd Bruggenjurgen, 2008 [60], which have been used in similar analysis [60,61]. Calculations were based on the measured utilities of a large German pilot project on acupuncture, which also examined patients with different allergic diseases, were incorporated [62]. Thus, for the present model, we used the following annual health state utilities: mild allergic rhinitis, 0.7579; moderate to severe allergic rhinitis, 0.7378; severe allergic rhinitis and mild allergic asthma, 0.7317; severe allergic rhinitis and moderate to severe allergic asthma, 0.6985; no symptoms, 0.7841; and death, 0.0.
Costing Methods
Since the analysis was conducted from the third-party-payer perspective, only direct medical costs which are reimbursed by EOPYY were considered in the model. The total reimbursement cost for each health state reflects and encapsulates all the possible healthcare resource consumption of patients with AR and AA during the 1-month cycles of the model (i.e. immunotherapy acquisition, hospitalization, physician visits, symptomatic medications, complementary, imaging tests, lab tests, and costs for the management of adverse events (such as stomatitis, infections of esophagus etc). Resource use associated with each health state was based on expert’s opinion and data from official sources (e.g. Government Gazette, Ministry of Health). The volumes of resource units were combined with the corresponding national unit costs to aggregate a total cost per for each health state. All costs applied in the analysis refer to the year 2018.
Drug acquisition and administration costs
The drug acquisition costs were calculated combining the drug dosing schedules with the corresponding reimbursed drug costs. The reimbursed drug costs depend onthe way each drug is provided through the healthcare system (hospital, EOPYY pharmacies, retail pharmacies) and the way these medicines are administered (IV, orally). In this context, when a drug therapy is administered through IV/SC/IM infusion only in the hospital setting (i.e. drug for severe asthma) (law N3816/2010), the payer is reimbursing the hospital price minus 5%. The reimbursed cost of drugs included in the drug positive lists (i.e. SABA, LABA, ICS), was calculated on grounds of the social security reimbursement price, defined by the internal reference price system attached to the latest published positive drug list (May 2018). In particular, when the drug retail price was higher than the corresponding reimbursement one, the payer cost was calculated based on the reference price minus the patient co-payment plus 50% of the difference between reference price and retail price in case where no generics exist in corresponding drug class. Otherwise, where generics exist, the difference between reference price and retail price is fully (100%) covered by the patient and, as such, no additional costs occur for the EOPYY. On the other hand, when the retail price is lower than the reference price, the payer cost is obtained from the retail price minus the patient co-payment, plus up to 50% of the difference between reference price and retail price. Hospital and retail prices were obtained from the latest price bulletin issued by the Greek Health Ministry in May 2018 [63].
The costs of disease management for patients in the each health state were calculated by multiplying the number of resource units (obtained from the literature) with the corresponding costs per unit (EOPPY reimbursement rates). Finally, the costs for the management of adverse events were also calculated by combining the volumes of the resources needed (based on expert’s opinion) with the corresponding unit costs. The aforementioned data were combined with the treatment specific incidence rates of the adverse events as provided from the literature. These costs were one-off costs for the treatment of patients.
Data Analysis
The cost-effectiveness of SCIT over the symptomatic treatment was evaluated by calculating the incremental cost per quality-adjusted life year (QALY) saved (ICER). For a treatment to be considered cost-effective a threshold of €35,000 per QALY was used in the current analysis. This is based on the WHO guidelines stating that a treatment should be considered cost-effective if the ICER is between 1 or 3 times the GDP per capita of that country and a treatment is considered highly cost effective at less than 1 times the GDP per capita [64]. The GDP per capita in Greece was estimated at €17,055 taken from the IMF estimation of GDP per capita using current prices [65].
Furthermore, one way sensitivity analyses were undertaken to test the robustness of the results, by varying individual parameters between low and high values within plausible ranges in order to ascertain the key drivers of cost-effectiveness. Nevertheless, the majority of input data used in the current model are subject to variation. Therefore, in order to deal with uncertainty, a probabilistic sensitivity analysis (PSA) was performed using a Monte Carlo simulation. In this analysis, probability distribution was assigned around each parameter (i.e. costs, utilities etc) and cost-effectiveness results associated with simultaneously selecting random values from those distributions were generated. In particular, utility values are constricted on the interval zero to one and hence they were varied according to beta distribution. The gamma distribution and the lognormal distribution were applied for the cost and effectiveness variables, respectively.
Then, 5,000 estimates of costs, QALYs, and incremental cost per QALY saved were obtained performing the bootstrapping technique. A cost-effectiveness acceptability curve was plotted, showing the proportion of simulations that are considered cost-effective at different levels of willingness to pay per QALY gained.
Deterministic Results
The model predicted that discounted quality adjusted survival of SCIT treated patients was higher compared to those treated with symptomatic treatment by 0.89 QALYs. Moreover, the total cost per patient for SCIT and symptomatic treatment was estimated to be €7,522 and €10,230, respectively.
Hence, use of SCIT leads to a cost saving of €2,708 over symptomatic treatment. One reason for this difference in the total cost between SCIT and symptomatic treatment was the treatment cost for AR (SCIT: 3,403€ vs symptomatic treatment: 5,633 €). Moving further, the symptomatic treatment regimen had significantly higher costs for treated AA symptoms (SCIT: 2,028 € vs symptomatic treatment: 4,597 €).
Based on the above, SCIT seems to be a dominant alternative over symptomatic treatment in a 10 year time horizon as the former is related with greater health benefit and lower total lifetime cost.
One-way sensitivity analysis is presented in Table 3 and Figure 2. For simplicity reasons, only the variables with significant impact in the ICER per QALY are presented. The one way sensitivity analysis indicates that the most important parameters in the analysis include: the reduction for the anti – allergic treatment due to symptomatic treatment and SIT, the discounting rate, the utilities weights, the AA treatment cost, the AR treatment cost, the remission rates of asthma per year and the SCIT cost.
Figure 2. One – Way Sensitivity Analysis, Tornado Diagram.
Nonetheless, notably most of them have marginal impact and only the reduction for the anti – allergic treatment due to symptomatic treatment affect significantly the results, however the maximum impact is €1,756 and the corresponding incremental cost per QALY raised at €-4,142 (Figure 2).
Probabilistic sensitivity analysis
The PSA confirmed the deterministic results. The cost-effectiveness acceptability curves (CEA) showed that at a WTP of €10,000, SCIT is dominant in any WTP threshold (Figures 3 & 4).
Figure 3. Cost effectiveness acceptability curve.
Figure 4. Cost effectiveness plane.
In times of major economic challenge, the best possible care should be determined by evaluating the effectiveness of treatments, by means of life years or quality-adjusted life-years (QALYs) gained, in conjunction with the respective long-term financial costs that these generate. In this context, a simple price comparison amongst comparators would be misleading, ignoring the overall economic impact on the health care system and society in general. Thus, cost-effectiveness and cost-utility analyses represent robust methodologies to quantify the benefits and to evaluate the trade-off amongst available treatments. Since, trials rarely collect enough data on treatment costs and consequences for rigorous economic assessment, mathematical modeling is required to support decision making in health policy. Used appropriately, modeling is a useful technique, particularly to extrapolate beyond trial duration.
Based on a Markov state model developed to evaluate SCIT and symptomatic treatment therapy in patients with AR and AA, the present analysis has provided a strong indication that the use of SCIT improves survival and patient quality of life. The parameters of the Markov model were based on clinical data, published estimates from the literature and experts’ opinion regarding the Greek healthcare setting. The analysis was made from a third-party payer perspective and the effectiveness measures were QALYs and LYs. According to the results, SCIT dominates symptomatic treatment alternative as it is associated with lower costs and higher clinical efficacy in both cases.
Our findings are in line with those presented in previous economic analysis comparing AIT as a treatment for patients with AR and AA [66-68]. In specific, a cost-utility study carried out in Germany [66] showed that SCIT is a highly effective treatment for treatment of AR and AA with an ICER of € 8.308 per QALY. In addition, a French study [68] showed that SCIT is an economically effective treatment in adult patients, especially with dust mite sensitization, with ICER of $ 393 per QALY and an economically effective treatment in adult patients (pollen) with ICER of $ 1,327 per QALY. Similar findings were obtained in a study conducted in selected EU countries including Austria, Denmark, Finland, Germany, Netherlands and Sweden[67], which showed that the implementation SCIT in patients with seasonal AR is clinically and economically documented.
To our knowledge, this is the first study aiming to evaluate the costeffectiveness of SCIT over symptomatic treatment in patients with AR and AA in Greece and is timely due to the recent financial crisis.
SCIT clinical studies show an improvement in allergic symptoms of AR and AA, by means of symptomatic treatment, hospitalization and quality of life [69-71], more so in patients with increases disease severity. Thus, SCIT is positively associated with improved survival and QoL rates and subsequently influence hard health outcomes, such as hospitalizations and decreased usage of symptomatic treatment.
Moreover, this model could be applied to all countries that would like to apply AIT as the dominant model for treating patients with severe AR and AA in order to improve patients' QoL and long-term financial benefit for the health systems
The analysis pursued has specific drawbacks and limitations. First of all, limitations in the model arise from the nature of the underlying data, which in several cases were not available with the required level of detail. In order to overcome this impediment, conservative assumptions were made. Moving further, based on medical expert’s opinion, the management of adverse events is limited in the Greek healthcare setting and this may have led to an underestimation of actual related costs. In addition, it should be noted that this study addresses only direct costs. However, there are indirect costs (which are very high) also involved, but due to the perspective adopted and the lack of data, economic benefits in this area will be considered separately. In addition, it needs to be acknowledged that the results presented here reflect a subgroup of patients and it is hard to extrapolate them more broadly. These areas could be taken into account for further research, which should have .sufficient size to ensure statistical power and collect information on long term hard outcomes such as hospitalization rates and increased use of symptomatic treatment. Finally, it should be noted that the results must be seen in the local context and based on the present time resource use and prices. If any of the underlying parameters used in the model change, the results and the conclusions of the analysis may also change. Also, the analysis confined to the health care system and the costs for payers. We did not consider wider financial and socioeconomic impacts, such as hospital visits in emergency room, which would probably allow direct and indirect costs savings for patients, through reduced transportation costs to and from the clinic, and reduced loss of income due to absenteeism from employment for patients and their relatives.
Clinical data were used together with local resource utilization and price data, to evaluate whether SCIT is cost-effective for the treatment of patients with AR with or without AA. Using conservative assumptions, the present economic evaluation suggests that SCIT provides significant health outcomes and is less costly compared to standard therapy. Hence, SCIT should be considered as a dominant intervention in the Greek healthcare setting with respect to reimbursement decisions by the National Organization of Healthcare Provision (EOPYY).
No any disclosures
Citation: C. Mylonas, N. Maniadakis, N. Kitsioulis, P. Xepapadaki, N. G. Papadopoulos. Economic Evaluation of Subcutaneous Allergen Immunotherapy versus Symptomatic Treatment in Patients with Allergic Rhinitis with or without Asthma. Health Econ Outcome Res Open Access , 2021, 7(1): 165 (011-017).
Received: 24-Oct-2019 Published: 26-Jan-2021, DOI: 10.35248/2471-268X.21.7.165
Copyright: © 2020 Mylonas, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.