Review Article - (2021) Volume 7, Issue 7
A year after since the identification of the SARS-CoV-2 virus and its genome, an exceptional effort by the scientific community has led to the development of over 300 vaccine projects. Over 40 are now undergoing clinical evaluation, ten of these are in Phase III clinical trials, and seven of them have ended Phase III with positive results and were granted Emergency Used Authorization (EUA) in the Philippines. Existing data suggest that new vaccine candidates may be instrumental in protecting individuals and reducing the spread of pandemic. The conceptual and technological platforms exploited are diverse, and it is likely that different vaccines will show to be better suited to distinct groups of the human population. Moreover, it remains to be elucidated whether and to what extent the capacity of vaccines under evaluation and of unrelated vaccines such as BCG can increase immunological fitness by training innate immunity to SARS-CoV-2 and pathogen- agnostic protection. Due to the short development time and the novelty of the technologies adopted, these vaccines will be deployed with several unresolved issues that only the passage of time will permit to clarify. A meta-analysis model is used to study vaccine allocation that aims to minimize deaths while satisfying group prioritization for immediate vaccination. It is our tenet that in the long run more than one vaccine will be needed to ensure equitable global access, protection of diverse subjects and immunity against viral variants.
Adverse Reaction; Efficacy; Pandemic; Philippine Health Guidelines; Literature Review
The coronavirus disease 2019 (COVID-19) is a novel beta-coronavirus that was initially identified to cause respiratory problems among people from a province in China in late 2019. It is a viral infection transmitted through respiratory particles, fomites, and other biological matter [1]. Beta-coronaviruses have caused outbreaks in the recent decades, with COVID-19 being characterized as a pandemic by the World Health Organization on the first quarter of 2020 [2].
The Philippines is one of the countries greatly affected by this pandemic [3]. Its government placed the country under community lockdown since March 2020 as a measure to control the spread of the virus [4]. The continuous lockdown contributed to the increase in unemployment rate (10.4 percent as of December 2020) [5] decrease in remittance inflow (14-20 percent) [6] and decrease in GDP (9.5 percent decline in 2020) [7]. Bringing back jobs is crucial for a sustainable recovery of the economy. All these will be possible when the COVID-19 spread is controlled.
Vaccination is considered to be an effective tool in preventing the spread of and deaths due to infectious diseases [8]. Vaccine development takes years to complete from clinical trials to manufacturing [9]. Vaccinating huge groups is another problem entirely. Vaccines have specific storage and shipment requirements to maintain its effectiveness [10]. Once vaccines are available for COVID-19, frontline workers should be vaccinated first followed by the elderly and people with preexisting conditions, according to various government protocols. The Philippine government plans to follow the same prioritization of people for vaccination but with the addition of indigenous people and uniformed personnel in the list [11]. The plan is also to prioritize those from the cities to expedite the recovery of the local economy [12]. Distribution of vaccines will be done through local government units (LGU). Since the vaccines are limited, proper allocation of doses for each LGU is important. An optimal allocation can be found by looking at the problem as an optimization problem that minimizes the number of deaths and follows the prioritization approved by the government. Technical problems connected with the production of billions of doses and ethical ones connected with the availably of these vaccines also in the poorest countries, are imminent challenges facing us.
The decision to select only case-control studies was determined by the fact that many studies of this type have been conducted in many parts of the world to evaluate the association between the efficacy of Covid-19 vaccine. Moreover, this systematic review aims to summarize the evidence of medication reviews as performed in clinical practice, irrespective of patient characteristics, setting and outcome measures.
Statement of the Problems
This study focuses on Covid-19 Vaccine literature review and meta-analysis. Specifically, it sought to answer the following questions.
1. What is the selection process of eligible studies for meta-analysis?
2. What are the characters of Covid-19 vaccines authorized for use by the Food and Drug Administration (FDA) in the Philippines?
3. Which vaccine do studies prefer for covid-19 vaccine pharmacokinetics in terms of:
a. Efficacy,
b. Adverse effect and
c. Contraindications
4. What are the trends of Covid-19 vaccine in terms of:
a. Effect size,
b. Heterogeneity,
c. Random Effect Model,
d. Forest Plot, and
e. Confidence Interval
What are the over-all implications of covid-19 vaccine use in the Philippines?
Significance of the Study
The study findings will provide the Department of Health (DOH) and Local Government Unit (LGU) as baseline data for the community on what specific actions are they going to take. More importantly, it will serve as an essential basis for policy-making and capability building for medical practitioner for appropriate evidence used in this new normal. Doing this may enable them to provide timely and relevant actions for the management and control of the pandemic.
The result of the study will also drive information awareness to the general public on the efficacy and pharmacokinetics and overall implication of covid-19 vaccine use.
Scope and Delimitations of the Study
The study was limited only to the development of the Meta-Analysis of the Covid-19 Vaccine in the Philippines specifically (BioNTech/ Pfizer, Moderna, Astra Zeneca, Janssen, Covaxin, Gamaleya, and Sinovac Biotech) approved by Food and Drugs Administration (FDA) based on their Emergency Used Authorization (EUA).
The variable focuses on the pharmacokinetics, trends, and implications of covid-19 vaccine in the Philippines.
Search Strategy
To identify studies on the association between covid-19 Vaccine, the researchers systematically searched the literature databases MEDLINE, Embase, and the Science Citation Index for relevant articles published year 2020 and beyond. The researchers used subject headings and keywords in English depending on the search structure of the literature database to combine the references related to the population, the exposure, and the disease. The search was not restricted by language filters and no date limits or other filters were used. A detailed description of the search strategy is provided in Figure 1. Included articles were also manually searched for potentially relevant citations not detected by the electronic search.
Data gathering procedures was considered as shown below guided with journals and articles for validations.
Reviewing and selecting articles of Inclusion
There are several strategies one can use to determine the inclusion or exclusion of a research. Simply reading the title of an article can help provide insight to whether or not it should be included, but can also lead an article to be excluded prematurely. Reading an entire article provides greater insight than the title, but is time consuming. Cooper suggests reading both the title and abstract as a more reliable source of determining inclusion or exclusion. The technique used in this analysis was a title and abstract review. After review of the title and abstract, a decision was made regarding whether or not to retrieve the full document. This approach enabled the reviewers to have a clearer understanding of the study.
On some occasions an abstract will not provide all necessary information or an abstract was not readily available. Therefore the decision will be made to be more inclusive then exclusive at this stage to avoid missing significant documents. After combining both students’ findings and excluding duplicates. The teaching strategy in science In/Out Form will be used during the next stage in the review to determine if articles should be included in the final analysis.
Apply Inclusion and Exclusion Criteria
The following inclusive criteria were set and reviewed by 2 independent investigators:
1. Study focusing on covid-19 vaccine;
2. Study method is based on a cohort study design;
3. Study followed the standard vaccination protocol of covid-19;
4. The study had to report correlations or other measures of association.
5. The study must be written in English.
The exclusion criteria were as follows:
1. Not published as a full text;
2. Cohort studies without defined groups of non-responders and responders, or the responder anti-HB threshold was no higher than 10 IU/L;
3. If study does not cover the vaccine seven types of vaccine.
If data were duplicated between studies, only the study most recently published with the larger number of participants was included.
Screening and Coding Process
A data coding form was be developed by the researcher to code articles for the meta-analysis. The design for the extraction form was based on (1) methodology used (2) participant information (3) study type. Methodological features provided details concerning methods used during the study and included: (a) Research design (experimental, descriptive, longitudinal, other); (b) Design (between subject, within subject, other); (c) Time frame (0-3 months, 4-6 months, greater than 6 months). Participant features included (n) Sex (male, female, combined); (d) Geographic location; and (e) Publication type (journal article, dissertation or thesis, other).
Content Validation for coding process
A request to validate and guide during the coding process from Dr. Raul C. Orongan, an expert and statistician from Central Mindanao University.
Recorded Variables for meta-analysis
For each multi section study, the recorded variables will include: author, year of publication/ year of submission, number of sections, data correlations, research titles, research objective, research methods, data collection instrument, data analysis techniques and summary of major findings of the study.
Dependent Variable
In this study, the dependent variable of interest will be the covid-19 vaccine efficacy and its overall implications to the Philippines
Independent Variable
The characteristics of the selected studies will be taken into account by several variables to be identified by the researcher during the coding procedure. Therefore, the researchers believe that those participants and experiments related variables might contribute to the variation of outcome measures. Eventually, the magnitude of heterogeneity in effect size estimates across studies might have occurred due to the variation in the sample and design characteristics of the experiments taken place on those selected studies. Hence, the potential effect of the independent categorical or moderator variables on the overall effect size estimates will also be investigated in this study.
Sample studies for data coding and meta-analysis
The sample of the study that can undergo data coding and analyze will be those who have appropriate criteria and meet standards for coding.
Research Ethics
This study utilizes publicly available studies and literature on COVID-19 Vaccines. Due reference was also made on studies and literature cited utilizing the APA 8th Edition guidelines.
Data Analysis on meta-analysis
After computing all weighted effect sizes, effect sizes will then be averaged over studies or sets of studies in the Comprehensive Meta-Analysis program. Moreover, effect sizes of all primary studies and subgroups within studies could be compared using homogeneity analyses to determine if they differed significantly.
Calculations on effect size
To make comparisons among studies possible, outcomes of each study were transformed to a general effect measure of the performance measure coded for each study, means and standard deviations, or, if not available, other outcome variables will be extracted from the coding scheme to compute weighted effect sizes based on the standardized mean difference Cohen’s d. Using the standardized mean difference, studies with unlike outcome measures are transformed into a comparably scaled outcome format. To account for differences in sampling error related to the sample size in different studies, the mean effect size d will be weighted by the variance of the sample. Furthermore, confidence intervals of 95% will determine the spread of scores around the mean effect size per study. In studies which reported statistically significant differences on the pretest despite of the random allocation of students or groups, the standardized mean difference will be calculated by dividing the difference scores by the within groups standard deviation. For studies with dependent samples not reporting the correlation between the pre- and the post measure, a correlation of 0.5 will be used to determine the variance, and the standard deviations of the posttests will also be used for standardization of the effect size. All calculations of the comparable effect sizes will be done through RevMan Version 3.5 software.
Homogeneity Analysis
To compare effect sizes from different (sets of) studies, we tested the homogeneity of the weighted effects using the Q statistic. Cochran’s Q refers to the weighted sum of squared differences between individual study effects and the pooled effect across studies. Thus, a significant p value of the Q statistic would show there to be large variation between the studies because the studies show more variation than would be expected by the standard errors. The homogeneity statistics (Q) will be used for (groups of) weighted effect sizes to determine whether the effect sizes varied statistically significantly, that is, whether the findings have shared a common effect size in the population. If Q will be not statistically significant, a fixed-effects model will be adopted for data analysis. If Q will be statistically significant, a randomeffects model will be used.
Fixed-effects model
A fixed-effects (FE) meta-analysis model (HedgesandVevea1998; Rice, Higgins, andLumley2018) is defined by (1); it assumes that different studies have different effect sizes (θ1 6=θ2 6=···6=θ K) and that the effect sizes are fixed quantities. By fixed quantities, we mean that the studies included in the meta-analysis define the entire population of interest. FE models are typically used whenever the analyst wants to make inferences only about the included studies. The target of interest in an FE model is an estimate of the weighted average of true study-specific effect sizes.
Random-effects model
A random-effects (RE) meta-analysis model assumes that the study effect sizes are different and that the collected studies represent a random sample from a larger population of studies. The goal of RE meta-analysis is to provide inference for the population of studies based on the sample of studies used in the meta-analysis.
Forest Plot
The usual way of displaying data from a meta-analysis is by a pictorial representation. This displays the findings from each individual study as a blob or square, 21 with squares towards the left side indicating the new treatment to be better, whereas those on the right indicate the new treatment to be less effective. The size of the blob or square is proportional to the precision of the study (roughly speaking, the sample size). A horizontal line (usually the 95% confidence interval) is drawn around each of the studies’ squares to represent the uncertainty of the estimate of the treatment effect. The aggregate effect size obtained by combining all the studies is usually displayed as a diamond.
Meta-regression
Meta-regression explores a relationship between the study-specific effect sizes and the study-level covariates, such as a latitude of a study location or a dosage of a drug. These covariates are often referred to as moderators. See, for more information about meta-regression. (Figure 1)
Selection Process of Eligible Studies
The selection process employed to identify eligible studies is displayed in Figure 1. A total of 132 studies were acquired from the PubMed, EMBASE, China National Knowledge Infrastructure (CNKI) databases and peer-reviewed medical journals. After reviewing the titles, abstracts and full text, we excluded 122 irrelevant studies. Finally, a total of 10 articles were included in the meta- analysis. The main characteristics of all of the eligible studies are shown in Tables 1 and 2. Furthermore, all these studies assessed the safety, acceptability, and efficacy of the vaccine at Phase III Clinical Trials. (Table 1)
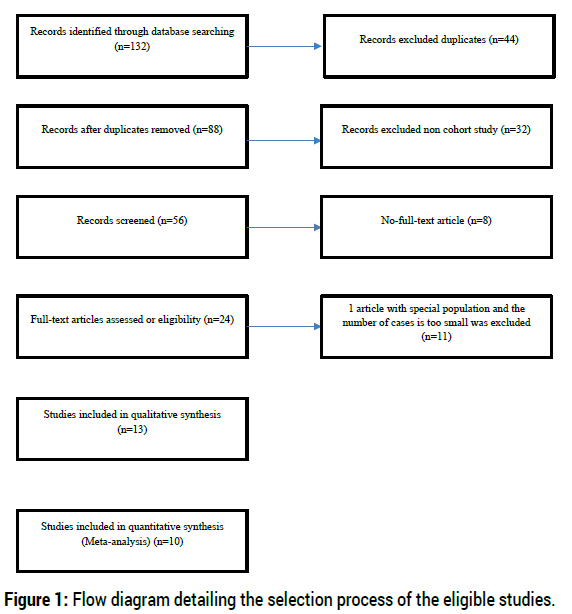
Figure 1: Flow diagram detailing the selection process of the eligible studies.
| Treatment Categories | N (No. of Studies) | Percentage (%) |
|---|---|---|
| BioNTech/ Pfizer | 20 | 15.15% |
| Moderna | 23 | 17.42% |
| University Oxford/ Astra Zeneca | 18 | 13.64% |
| Janssen/Johnson & Johnson | 15 | 11.36% |
| Covaxin | 20 | 15.15% |
| Gamaleya Nat. Center of Epidem. and Microbiol | 20 | 15.15% |
| Sinovac Biotech | 16 | 12.12% |
| TOTAL | 132 | 100% |
Table 1: Frequency Distribution of Studies on Covid Vaccine.
Table 1 shows the Frequency Distribution of Studies on Covid Vaccine, date revealed that Moderna has the highest number of studies which is 23 (17.42%). BioNTech/ Pfizer, Covaxin and Gamaleya has 20 (15.15%). Followed by University Oxford/ Astra Zeneca has 18 (13.64%). Then Sinovac Biotech has 16 (12.12%) . Lastly, Janssen/Johnson & Johnson has 15 (11.36%). (Table 2)
Table 2 shows the summary of references included in the meta-analyses based on the inclusion exclusion criteria. Among the included studies, only one study was conducted in the Philippines, which tackles mainly on the allocation of vaccines.
| Year | Title (Reference) | Author | Journal | Subject Area | Key Terms |
|---|---|---|---|---|---|
| 2021 | COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines | Meo | European Review for Medical and Pharmacological Sciences | 1 | H |
| 2021 | Principles and Challenges in anti-COVID-19 Vaccine Development | Strizova | International Archive of Allergy and Immunology | 1 | H |
| 2021 | Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort | Geisen | Ann Rheum Dis Epub | 1 | H |
| 2021 | COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials | Yan | Journal of Pharmaceuticals | 1 | H |
| 2021 | What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2 | Hodgson | Journal of Pollard Medical Science | 1 | H |
| 2021 | COVID-19 Vaccination Efficacy and Safety Literature Review | Halim | Journal of Clinical Medical Research | 1 | H |
| 2021 | Looking beyond COVID-19 vaccine phase 3 trials | Kim | Nature Research 2021 | 1 | H |
| 2020 | Double-Blind, Randomized, Placebo- Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac – PROFISCOV: A structured summary of a study protocol for a randomised controlled trial | Palacios | Springer Nature | 1 | H |
| 2021 | Current State of the First COVID-19 Vaccines | Prub | Vaccines 2021 | 1 | H |
| 2021 | Optimal Allocation of COVID-19 Vaccines in the Philippines | Buhat | Medical Research Journal | 1 | H |
| 2020 | Drug treatments for covid-19: living systematic review and network meta-analysis | Siemieniuk | British Medical Journal | 1 | H |
| 1- Medicine; 2- Agricultural and Biological Sciences; 3- Nursing; 4- Biochemistry, Genetics, and Molecular Biology; 5- Immunology and Microbiology; 6- Chemical Engineering; 7- Engineering; 8- Environmental Science; MA: Management Accounting; NE: Nutrition Education; H: Healthy. | |||||
Table 2: Summary of References included based on inclusion exclusion criteria.
The reviewed literature offers definitions for the basic concepts of management accounting for healthy nutrition education. Some reflections of the concepts applied in the context of this research are incorporated, which have shaped this field. To avoid interpretations, the basic concepts of this subject have been defined and these will be used in the development of the study. Data revealed that most of the journals taken for meta-analysis belongs to medicine journal and health. Most of the studies published were also conducted outside the Philippines under the foreign context as the main consideration. This implies that data on covid-19 vaccine trends in the Philippines is limited. This may account to the differing stand of the general populations’ intent to get vaccinated. With vaccination as the most effective way to combat the pandemic [13], it is imperative that the public should be informed of the latest trends and development on COVID-19 vaccines. Public information based on scientific and non-biased results should be carefully disseminated to the population to ensure high acceptance of newly-introduced vaccines. Otherwise, there is a risk of disseminating counter-productive messaging that may reinforce hesitancy in those already hesitant [14].
Characters of COVID-19 Vaccines Authorized for Use by the FDA
Table 3 shows the Characters of Covid-19 Vaccine. Data shows that BNT162b1/ BNT162b2 also known as BioNTech/ Pfizer was produced by Germany/US and has the mechanism of mRNA; mRNA-1273 (Moderna) was manufactured by US and has the mechanism of mRNA; AZD1222 (University Oxford/ Astra Zeneca) was made by UK and has the mechanism of Adenovirus vector, chimpanzee; Ad26.COV2 (Janssen) is the product of Netherland and has the mechanism of Adenovirus vector, Ad26; (Covaxin) is the output of India and has the mechanism of Whole virion Inactivated Corona Virus Antigen; Sputnik V also known as Gamaleya was produced by Russia and has the mechanism of Adenovirus vectors; CoronaVac (Sinovac Biotech) is made by the China has the mechanism of Inactivated SARS CoV-2.(Table 3)
As reflected in Table 3, vaccines differ mainly in the platforms used, mechanism of actions, storage, and transport mechanism. Platforms used by vaccines approved for use in the Philippines belong to three major classification namely: mRNA, viral vectors, and inactivated virus which consequently dictates the mechanism of actions of the vaccines. The mRNA vaccines on one hand, such as Pfizer/BioNTech and Moderna works by delivering mRNA segment that codes for the S-peptide of SARS-CoV-2 to trigger the expression of the SARS-CoV-2 antigen. AstraZeneca, Janssen, and Gamaleya vaccines on the other hand uses an adenovirus vector to deliver DNA of SARS-CoV-2 to the cell that elicits an immune response against COVID-19. Finally, inactivated virus vaccines like Sinovac and Covaxin is constitute of a physiochemically treated virus to inhibit pathogenicity, which when delivered into the body will be engulfed by APCs to trigger immune response.
Another main difference is the storage and transport mechanism wherein some vaccines require specialized structures and super low temperature to maintain its viability such as Pfizer/BioNTech while other vaccines such as Sinovac and Covaxin may be stored in regular refrigerator and storage facilities available in developing countries like the Philippines.
Table 3 also shows that vaccines granted with EUA in the Philippines are the same in terms of age group for vaccination and route of administration. Vaccines may also be given as either two shots or single dose. One key factor that should be considered however is the safety and efficacy of the vaccines based on data derived from their Phase III Clinical Trials. The goal of vaccine development is to obtain direct evidence of vaccine efficacy in protecting humans against SARS-CoV-2 infection and COVID-19. A candidate vaccine against SARS-CoV-2 might act against infection, disease, or transmission, and a vaccine capable of reducing any of these elements could contribute to disease control [15].
| Characteristics | Pfizer/ BioNTech | Moderna | AstraZeneca | Janssen | Novavax | Gamaleya | Sinovac |
|---|---|---|---|---|---|---|---|
| General Name | Pfizer/BioNTech Vaccine1 | Moderna Vaccine | ChAdOx1 nCoV-19 | Ad26.COV2.S | Whole Virion Inactivated Corona Virus Vaccine | Sputnik V Gam-COVID-Vac COVID-19 Vaccine | SARS-CoV-2 Vaccine (Vero Cell) Inactivated (CoronaVac) |
| Generic Name | Tozinameran, brand name Comirnaty | Moderna COVID-19 | COVID-19 Vaccine AstraZeneca | Janssen COVID-19 Vaccine | COVAXINTM | Gamaleya Sputnik V | CoronaVac |
| Manufacturer | Pfizer, Inc and BioNTech | ModernaTX, Inc | Catalent Anagni S.R.L., Anagni (FR), Italy | Grandriver Aseptic Manufacturing Inc., Janssen Pharmaceutica NV, Janssen Biologics | Bharat Biotech International Ltd | Gamaleya National Center of Epidemiology and Microbiology | Sinovac Life Sciences Co., Ltd. |
| Type of Vaccine | mRNA (BNT162b2) | mRNA (mRNA-1273) | Viral Vector (recombinant, replication-deficient adenovirus vector) | Viral Vector (non-replicating) | Whole Virion, Inactivated Virus | Viral Vector (non-replicating) | Inactivated Virus |
| FDA Approval | Emergency authorization Dec 11, 2020 | Emergency authorization Dec 18, 2020 | Emergency authorization January 28, 2021 | Emergency authorization dated April 19, 2021 | Emergency authorization dated April 19, 2021 | Emergency authorization March 19, 2021 | Emergency authorization February 22, 2021 |
| Dose | Each dose contains 30 µg (0.3 mL) | Each dose contains 50 µg (0.5 mL) | 0.5 mL | 0.5 mL | Single human dose (o.5 mL) contains 6 µg active corona virus antigen (Strain NIV-2020-770) | 0.5 mL of component I and o.5 mL of component II | 600 SU/0.5 mL |
| No. of Injections | 2 shots, given 21 days apart1 | 2 shots, given 28 days apart | 2 shots with the second dose to be administered within 4 to 12 weeks after the first dose | Single shot | 2 shots, given 28 days apart | Administered in 2 stages, first of Component 1 then Component II 21 days after | Emergency Vaccination = 2 doses at 2 weeks apart; Routine immunization = 2 doses at 1 month interval |
| Intramuscular (IM) Injection in the deltoid muscle | |||||||
| Route of Administration | Intramuscular-deltoid muscle | Intramuscular-deltoid muscle | Intramuscular (IM) Injection in the deltoid muscle | Intramuscular (IM) Injection in the deltoid muscle | Intramuscular Injection (IM) | Intramuscular (IM) Injection in the deltoid muscle | |
| Age group of Vaccination | 16 years of age and older | 18 years of age and older | 18 years of age and older | 18 years of age and older | 18 years of age and older | Over the age of 18 | Clinically healthy persons aged 18 – 59 years old |
| Efficacy | 95% in preventing the SARS-COV-2 infection1 | 94.5% in preventing the SARS-COV-2 infection | 76% | 28 days after inoculation, it was found to have and efficacy of 85.4% against severe disease and hospitalization. | 81% interim efficacy | 91% interim efficacy | 78% in preventing the SARS-CoV-2 infection |
| Approximate cost per dose | Freely available in many developed na- tions. However, $19.50 per dose exclud- ing taxes, distribution, storage and health care services cost or approximately Php 2,379.00 | Free available in many developed nations. However, $32-37 per dose excluding taxes, distribution, storage and health care services cost or approximately Php 3,904 – 4,504.00. | Approximately Php 610.00 | $10 dollars per dose | ?400 to ?600 for country state and up to ?1200 for private hospitals; in the Philippines, it is estimated to cost Php. 854.00 per dose. | Cost is less than $10 per dose or around Php. 1,220.00 | $10.13 or around Php. 3,629.00, non-negotiated amount |
| Store at +2°to +8°, do not freeze. Discard if frozen. | Store in a dark place at a temperature not exceeding minus 18°C. Storing a thawed product in 0.5 mL vials (ampoules) is not allowed! The thawed drug can be stored in 3.0 mL for 2 hours at most. Re-freezing is not allowed. | Store at 2 to 8°C. Protect from light. Do not freeze. Shelf life is 12 months. | |||||
| Storage | Multiple dose vials are stored between -80oC and -60oC (-112oF to -76oF). Thaw and store undiluted vials in the refriger- ator [2°C to 8oC (35°F to 46°F)] for up to 5 days (120 hours). For immediate use thaw undiluted vials at room temperature [up to 25°C (77°F)] for 30 minutes. Un- diluted vials may be stored at room tem- perature for no more than 2 hours | Multiple-dose vials are stored between | Store in a refrigerator (2° to 8°C). Do not freeze. Keep vials in outer carton to protect from light. Once removed from the fridge may be stored between 2 to 25 degrees Celsius for up to 6 hours. | Store in a refrigerator at 2°C to 8°C for a single period up to 3 months, not exceeding the original expiry date. | |||
| -25° and -15°C (-13°to 5°F). Vials can be stored refrigerated between 2°C and 8°C (36° to 46°F) for up to | Open vials should be used as soon as possible and within 6 hours when kept between 2-8 degrees C | ||||||
| 30 days prior to first use. After the first dose has been withdrawn, vial should be held between 2°and 25°C (36° to 77°F), discard the vial after 6 hours | |||||||
| Transportation/ Distribution | Complicated, difficult distribution par- ticularly in low-income and hot climate countries. | Complicated, difficult distribution particularly in low-income and hot climate countries | To be transported at temperatures 2° to 8°C. | Vials that have not been punctured may be kept at 9° and °C for a total of 12 hours. Requires a specified DDL for easy monitoring of temperature. | Ideal for hot and humid countries. Do not require special freezer for storage. Equipped with Vaccine Vial Monitor (VVM7) which is a time-temperature sensitive dot that warns the end user when exposure to heat is likely to have degraded the vaccine. | Transported at temperatures not exceeding 180C. Shelf life is around 6 months. | To be transported at temperatures 2°to 8°C. Ideal for countries with no special transport equipment |
| Mechanism of Action | The vaccine is formulated in lipid parti- cles, which enable delivery of the RNA into host cells to allow expression of the SARS-CoV-2 S antigen. It elicits an immune response to the S antigen, which protects against COVID-19 |
The nucleoside-modified mRNA vaccine is formulated in lipid particles. It enables delivery of the nucleo- side-modified mRNA into host cells to allow expres- sion of the SARS-CoV-2 S antigen. The vaccine elicits an immune response to the S antigen, which protects against COVID-19. The antibodies are specific to the SARS-CoV-2 virus, to protect against a future infection | A monovalent vaccine composed of a single recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1) vector encoding the S glycoprotein of SARS- CoV-2. Following administration, the S glycoprotein of SARS-CoV-2 is expressed locally stimulating neutralizing antibody and cellular immune responses. | The vaccine is composed of a recombinant, replication-incompetent human adenovirus type 26 vector that, after entering human cells, expresses the SARS-CoV-2 spike (S) antigen without virus propagation. An Immune response elicited to the S antigen protects against COVID-19. | It is an inactivated vaccine obtained from SAR-CoV-2 strain. Used along with immune stimulants Alhydroxiquim-II to improve immune response and longer-lasting immunity. | Is a combined vector vaccine based on rAd type 26 (rAd26) and rAd type 5 (rAd5) – both of which carry the gene for SARS-CoV-2-full-length glycoprotein S (rA26S and rAd5-S). Adenoviral vectored-delivered antigens are known to induce both cellular and humoral immunity after a single immunization. | Constituted by a virus treated physiochemically to inhibit its pathogenicity. Upon injection, the inactivated viruses are engulfed by APCs and different epitopes are presented to the immune system. Rodriguez-Coira & Sokolowska, 2020) |
Table 3: Characters of COVID-19 Vaccines.
Even though the covid-19 vaccines are already distributed and used across the globe, still further research is needed to determine, whether shots will be required over the year to maintain immunity, or to be given annually like the flu shot. (Table 4)
Table 4 shows the pharmacokinetics of Covid-19 vaccines used in Philippines. Data revealed that for vaccine efficacy, Pfizer BioNTech has 95% efficacy rate, followed Moderna with 94.5% efficacy rate; Gamaleya (91%), Jenseen (85.4%), Covaxin(81%), Sinovac (78%) and Astra Zeneca (76%).
| Vaccines | Efficacy | Contraindications | Adverse Effect |
|---|---|---|---|
| Pfizer BioNTech | 95% in preventing the SARS-COV-2 infection[1] | - had a severe allergic reaction after a previous dose of the vaccine - had severe allergic reaction to any of the ingredients of the vaccine |
- Short-term mid-to-moderate pain at injection site - Fatigue - Headache - Muscle pain - chills - joint pain - nausea - feeling unwell - swollen lymph node - non-severe allergic reactions - diarrhea, vomiting - arm pain |
| Moderna | 94.5% in preventing the SARS-COV-2 infection | - hypersensitivity to any of the vaccine components | - Pain, erythema, or swelling on the injection site - Auxillary lymphadenopathy - Fever - Headache |
| Astrazeneca | 76% in preventing the infection | - Hypersensitivity to any of the ingredients of the vaccine. - Those who have experienced major venous or arterial thrombosis in combination with thrombocytopenia. |
- Injection site pain and tenderness - Fatigue - Headache - Feverishness - Myalgia, malaise - Pyrexia, chills - Arthralgia, nausea |
| Janssen | 28 days after inoculation, it was found to have and efficacy of 85.4% against severe disease and hospitalization | - Severe allergic reaction to any of the ingredients. | - Injection site pain - Redness of the skin and swelling - Tiredness - Headache - Muscle pain - Chills - Fever - Nausea |
| Covaxin | 81% interim efficacy | - Hypersensitivity to any of the constituents of the vaccine. - Pregnant and lactating mothers. - During fever or severe infection. - Individuals below 18 years old. |
- Headache - Fatigue - Fever - Body ache - Abdominal pain - Nausea - Vomiting |
| Gamaleya | 91% interim efficacy | - Hypersensitivity to the vaccine components. - Acute infectious and non-infectious diseases, flares of chronic disease - Pregnancy and breastfeeding. - Age underv18 years of age. Component II -Severe post vaccination complications |
- Pain on the injection site - Hyperthermia - Swelling |
| Sinovac | 78% in preventing the SARS-CoV-2 infection | - With known allergies to any of the components - febrile, patient in acute illness period and acute attack of chronic diseases |
- Local lymphadenopathy at injection site - Possible allergic reaction such as hives, allergic rashes and purpura, anaphylactic shock - Convulsion |
Table 4: Pharmacokinetics of Covid-19 vaccines used in Philippines.
Moreover, most of the covid-19 vaccines used in the Philippines have the same contraindications and reported adverse effect at phase III. Though these efficacies imply that the vaccines offer considerable protection against the infection, further research and evaluation should go on to attain higher efficacies while addressing any safety concerns that may go beyond local and systemic reactions that occur on patients after vaccination [16]. (Table 5)
In the meta-analysis, all Covid-19 Vaccine presented in Table 5 exhibited a significant positive influence (p < 0.05) in terms of their mean effect size on efficacy. The weighted mean effect size of the treatment categories ranges from 0.50 to 1.80. Using the random-effect model, the Janseen (d=1.783) has the largest mean effect size, followed by Gamaleya (d=1.276) and Moderna (d = 1.268) which are close in effect values. Nearly of the same effect values shared by Covaxin (manipulative, model and multiple representations) (d=1.072), Sinovach Biotech (d=1.035) and BionTech/Pfizer (0.687), then Astra Zeneca that still has positive effect level (d=0.933). Combination efficacy showed to have a moderately positive effect on the Covid-19 vaccine efficacy.
| Treatment Categories | N (Sample Size) | Mean Effect Size (Cohen’s d) | Lower Limit | Upper Limit | Z Value | P Value |
|---|---|---|---|---|---|---|
| BionTech/Pfizer | 130 | 0.687 | 0.332 | 1.041 | 3.797 | 0.000* |
| Moderna | 594 | 1.268 | 0.612 | 1.924 | 3.79 | 0.000* |
| Astra Zeneca | 484 | 0.933 | 0.118 | 1.748 | 2.245 | 0.000* |
| Janseen | 828 | 1.783 | 0.118 | 2.449 | 5.253 | 0.025* |
| Covaxin | 537 | 1.072 | 0.587 | 1.557 | 4.333 | 0.000* |
| Gamaleya | 257 | 1.276 | 0.186 | 2.365 | 2.295 | 0.000* |
| Sinovac Biotech | 164 | 1.035 | 0.377 | 1.694 | 3.083 | 0.022* |
| p < 0.05 | ||||||
Table 5: Effect of Treatment Categories on Covid Vaccine.
As presented in Figure 2, it can be observed that there are some interesting results obtained from the study. First, it can be observed in the case of Jenseen vaccine, it has the widest range of interval and yet possessed a large over-all effect size. A wider confidence interval may be a function of a small sample size, as well as inaccuracy in the measurement. Hence, it is established that this category contained studies with small sample size. Obtaining such result most likely stems from a bias against non-significant findings included, it likely results in meta-analysis overestimating effect sizes [17].
Second, the outcome of the Moderna Vaccine contradicts with the interpretation of confidence interval, where there is a wider interval and possessed the largest effect size, which is supposed to have fine interval. This case indicates that the category contained individual studies with small sample size that eventually affect the interval values surrounding the effect size. Sample size is one of the factors identified that can influence the magnitude and direction of effect size [18]. There is a negative correlation between sample size and effect sizes in studies included in meta-analysis [17]. Mollon et al. as cited by [18] stressed that when mentioning a similar scenario, the confidence intervals surrounding the over-all estimated effect size is still considered to be larger even when smaller studies are Janssen and moderna has greater directive impact on covid-19 vaccine efficay. (Figure 2)
BionTech/Pfizer, though was found to be one of the least studied categories, yet it presented a large effect on performance based on the analysis. The findings presented in comparison to traditional Trials, BionTech/Pfizer’s effect on covid vaccine efficacy is relatively higher. This is in line with the findings of [19] that “BionTech/Pfizer displayed a strong positive effect” towards efficacy in Covid-Vaccine.
In terms of Astra Zeneca, a recent meta- analysis mentioned by Copar states that examined virtual manipulative obtained a moderate effect size of 0.44 as compared to other treatments. In the same way, the meta-analysis conducted by Schroeder CM et al. [20] gave positive medium effect of manipulation strategies on covid vaccine. The positive effect size obtained in this study indicates that when using this effectivity in community. This study suggests that medical practitioners may opt with explaining the concept of covid vaccine by using maps or graphs, videos, images, models, materials, etc. to increase community’s conceptual understanding resulting to outstanding efficacy of covid-19 vaccine.
It is believed that if community that are exposed in an idea on vaccination program where they can actively relate the new normal settings to their interests and needs and present knowledge and have an opportunity to experience various meaningful experiences would be more meaningful. (Table 6)
| Topic/Variable | Outcome/ Analysis | Effect Measure | No. of Studies | Heterogeneity Test | I2(95% CI | ||
|---|---|---|---|---|---|---|---|
| Q | df | P | |||||
| Covid-19 Vaccine Efficacy | Odds Ratio | 20 | 55.9 | 54 | 0.40 | 0.6299 to 0.7441 | |
| Moderna | Covid-19 Vaccine Efficacy | Odds Ratio | 23 | 39.5 | 32 | 0.17 | 1.219 to 1.317 |
| Astra Zeneca | Covid-19 Vaccine Efficacy | Odds Ratio | 18 | 179.9 | 134 | 0.005 | 0.9225 to 0.9435 |
| Janseen | Covid-19 Vaccine Efficacy | Odds Ratio | 15 | 40.2 | 179.9 | 0.0004 | 1.775 to 1.791 |
| Novavax | Covid-19 Vaccine Efficacy | Odds Ratio | 20 | 15.9 | 40.2 | 0.007 | 1.022 to 1.122 |
| Gamaleya | Covid-19 Vaccine Efficacy | Odds Ratio | 20 | 12.44 | 15.9 | 0.09 | 1.253 to 1.299 |
| Sinovac Biotech | Covid-19 Vaccine Efficacy | Odds Ratio | 16 | 38.6 | 12.44 | 0.07 | 0.9337 to 1. 093 |
Table 6: Heterogeneity Statistics for Examples of Meta Analyses from the Literature. Meta-Analysis was conducted using either meta or meta in STATA.
A systematic review of clinical trials of Covid-19 vaccine patients concluded that vaccines may increase mortality. These studies had no inconsistency in risk ratio estimates (I2=0%) and a narrow uncertainty interval. Table 2 shows the heterogeneity statistics for risk differences as well as for risk ratios. Seven trials with no deaths in either treatment group do not contribute information on risk ratios, but they all provide estimates of risk differences. Using P values to decide which scale is more consistent with the data is inappropriate because of the differing numbers of studies. I2 values may validly be compared and show that the risk differences are less homogeneous, as is often the case.
A naive categorization of values for I2 would not be appropriate for all circumstances, although we would tentatively assign adjectives of low, moderate, and high to I2 values of 25%, 50%, and 75%. Figure 2 shows that about a quarter of meta-analyses have I2 values over 50%. Quantification of heterogeneity is only one com- ponent of a wider investigation of variability across studies, the most important being diversity in clinical and methodological aspects. Meta-analysts must also consider the clinical implications of the observed degree of inconsistency across studies. For example, interpretation of a given degree of heterogeneity across several studies will differ according to whether the estimates show the same direction of effect.
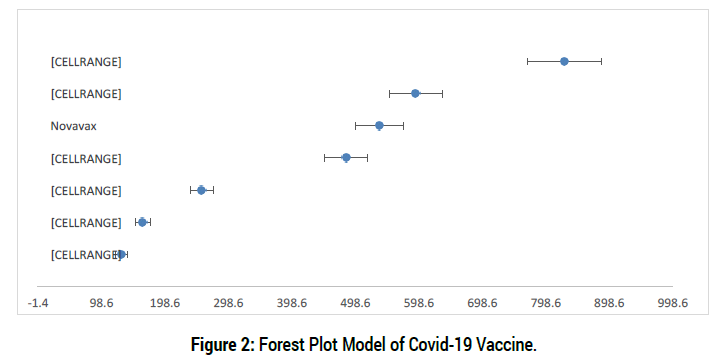
Figure 2: Forest Plot Model of Covid-19 Vaccine.
An alternative quantification of heterogeneity in a meta-analysis is the among-study variance (often called I2), calculated as part of a random effects meta-analysis. This is more useful for comparisons of heterogeneity among subgroups, but values depend on the treatment effect scale. We believe, I2 offers advantages over existing approaches to the assessment of heterogeneity (box). Focusing on the effect of heterogeneity also avoids the temptation to perform so called two stage analyses, in which the metaanalysis strategy (fixed or random effects method) is determined by the result of a statistical test. Such strategies have been found to be problematic. We therefore believe that I2 is preferable to the test of heterogeneity when assessing inconsistency across studies. (Table 7)
Using the random-effects model the computed mean effect size for 10 studies is 1.208, SE=0.115 as seen in Table 7 at a confidence level of 0.05, the 95% confidence limit was determined. The confidence limits were 0.984 to 1.433 that implies the population effect size is zero can be decline. Confidence intervals of continuous measures that include zero represent non-significant results [18]. This shows that the mean effect size of Efficacy of Covid-19 Vaccine is high and positive.
| Over-all effect | Mean ES | Mean SE | Lower limit | Upper Limit | Z-Value | P-value |
|---|---|---|---|---|---|---|
| 10 Studies | 1.208 | 0.115 | 0.984 | 1.433 | 10.534 | 0.000* |
| p<0.05 | ||||||
Table 7: Meta-analysis for all Studies.
Overall Implication of covid-19 vaccine use in the Philippines
The covid-19 pandemic has indubitably been be a great challenge to every Filipino in the part the covid-19 pandemic has unquestionably posed a significant challenge to any Filipino in the second half of the year. Although this extraordinary situation has triggered significant changes in our citizens' daily lives, we have shown our willingness to move forward in the face of adversity. Our strategy over the last year has been to ensure that the minimum public health requirements are met through the BIDA Solusyon campaign.
The dedication of scientists and medical professionals around the world has encouraged us to enter the new year with not only fresh perspectives, but also renewed hope and vigor in our battle against the pandemic. Breakthroughs in the production of a COVID-19 vaccine are a good way to protect our families by lowering the risk of serious cases. Countries all over the world are now implementing prospective vaccine campaigns to combat the pandemic's ideas, with the Philippines among those who have provided the go-ahead for the biggest vaccination campaign to date.
The Philippine government, through the National Vaccine Development Plan, brings together all national agencies, local government counterparts, as well as private sector and civil society partners. We will ensure the effectiveness of the national vaccine deployment program in providing secure, affordable, and usable vaccines for all Filipinos by approaching the vaccination program from a whole-of-system, whole-of-government, and whole-of-society perspective.
As our history, experience, and science has proven in the past decades, vaccines save lives. An effective and national vaccination programs, in tandem with the continued observance of the minimum public health standards, will pave the way for the recovery of our beloved country and bring us one step closer to our vision of a Healthy Pilipinas.
Altogether, the newly developed COVID-19 vaccines come with promise for a future that is brighter than 2020, but also with challenges. Two of the discussed vaccines received EUA by the FDA and one of them is expected to receive EUA status soon. The Philippines have approved several of the vaccines. The year 2021 will show how these vaccines will be rolled out and whether the desired goal of controlling the COVID-19 pandemic will be accomplished.
COVID-19 vaccine allocation is an immediate concern globally. A meta-analysis model is used to study vaccine allocation that aims to minimize deaths while satisfying group prioritization for immediate vaccination. Various approaches were studied, all of which assumed using a vaccine with 90% effectiveness rate to be used for at most 50% of the population. Various vaccines were also studied in terms of their associated costs and effectiveness, and determined that the vaccine with 89.9 % effectiveness and 183 PHP price per dose results to the lowest projected deaths. We then compared our result to various model variations and common allocation approaches, upon which our model achieved both optimal and a more equitable allocation.
The DOH, IATF, LGU and other concern organization may employ wellorchestrated dissemination information to up-scale acceptance of population and strengthen their intention to get vaccinated. Community may take positive action and be responsive towards government programs on the application of covid-19 vaccination action plan. Medical practitioners may conduct further studies and review literature to establish reliable baseline data on covid-19 safety and efficacy and researchers may add variables concerning public opinion and intent for the vaccination.
Citation: Cababan, Mc Arthur L, Catane, Gretchen V, and Wilfred G. Alava. Covid-19 Vaccines in the Philippines: A Meta-analysis. Health Econ Outcome Res Open Access, 2021, 7(7): 179.
Received: 23-May-2021 Published: 28-Jul-2021, DOI: 10.35248/2471-268X.21.7.179
Copyright: © 2021 Cababan, Mc Arthur L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.