Case Report - (2023) Volume 9, Issue 3
Parkinson’s Disease (PD) is a central nervous system disorder characterized by movement abnormalities such as tremors, rigidity, and gait freezing. The major hypothesis for the cause of PD is the reduction in dopaminergic neurons in the substantia nigra, a region in the middle of the brain. The main association tract connecting the substantial nigra to the spinal cord and motor cortex is called the corticospinal tract. The corticospinal tract has been identified to have impairment in PD patients and most neuromuscular disorders. An area of research that is being established and expanded upon for the treatment of PD is noninvasive brain stimulation using electrical stimulation with pulses called Transcranial Pulsed Current Stimulation (tPCS). A novel form of tPCS is being developed named Amplitude-Modulated tPCS (am-tPCS) where the polarity of the pulsed current being delivered between the two electrodes is being reversed over time from positive to negative and vice-versa. We evaluated stimulation of a 76-year old female with Parkinson’s Disease and resting tremor along the corticospinal tract with am-tPCS while using video analysis to compare the tremor pre and post stimulation over the course of five days. At the end of the five-day period there was an 80% reduction in tremor amplitude and a 73.5% reduction in the Unified Parkinson’s Disease Rating Scale (UPDRS) score.
Parkinson’s disease • Transcranial electrical transcranial current stimulation • Pulsed current stimulation • Tremor• Video analysis
Parkinson’s Disease is a common neurodegenerative disorder affecting patients in large numbers throughout the world [1-3]. It is mainly caused by progressive damage to the brain over many years, the condition shows symptoms of spontaneous shaking of body parts of the body, decelerating movement and stiffness in muscles [4]. People suffering from Parkinson’s also experience depression and anxiety. They experience loss of balance, sleep, and memory. It remains the second most common neurodegenerative disorder and most common movement disorder. Its onset is usually seen in people older than 60 years of age [4,5]. An area of research that is being established and expanded upon for the treatment of PD is noninvasive brain stimulation using electrical stimulation [6,7]. Otherwise known as Transcranial Current Stimulation (TCS), this research area studies how weak electrical current passes through a given brain region of interest to modulate neuronal activity. There are many different forms of tCS depending on what waveform one chooses to use to stimulate. These different forms are named after the shape of the electrical waveform which includes direct, alternating, random-noise, and pulsed. Each waveform has a different mechanism of action on a neuronal level which can lead to different treatment outcomes for PD. Furthermore, predicting the impact of the waveform parameters can allow a physician to personalize and optimize treatment procedures on a case-by-case basis. Transcranial direct current stimulation (tDCS) sends one small continuous pulse through two electrodes positioned on the head and does not induce cerebral activity directly but rather is focused on subthreshold modulation of neuronal membranes to alter spontaneous brain activity [8]. The membrane voltage gradient values induced by standard 2 mA-4 mA noninvasive direct current stimulation is <1 mV/mm which is insufficient in inducing effective spike firing in a neuronal population [9]. For Transcranial Alternating Current Stimulation (tACS), a sinusoidal pulse is sent between two electrodes on the head. tACS is like tDCS in the fact that it does not induce cerebral activity directly and that its induced voltage gradient is<1 mV/mm. However, tACS focuses on entraining endogenous oscillations by guiding the spike frequency rate and direction of a single neuron to large neuronal tracts rather than the excitation/inhibition of a region of interest through anodal/cathodal direct current stimulation [10-12]. Just like tACS, Transcranial Random Noise Stimulation (tRNS) uses another sinusoidal current between two electrodes such that the frequency of the sinusoidal current is randomized between a predetermined range. The specific mechanism of actions of tRNS are currently being established and it has been observed so far that repeated opening and closing of sodium channels in a neuronal ensemble generated by a random frequency field leads to robust excitability in the targeted region of interest [13]. Furthermore, due to the random distribution of frequencies, it is speculated that tRNS induces a temporal summation of charge which leads to larger voltage gradients>1 mV/mm [14, 15]. For Transcranial Pulsed Current Stimulation (tPCS), there are two major mechanisms of action: pulsed current can summate charge to induce higher voltage gradients along a neuronal ensemble and pulsed current stimulation can induce phasic subthreshold modulation along a region of interest [16, 17]. First, this induction of higher voltage gradients (>1 mV/mm) from charge current summation is achieved by sending highfrequency pulses faster than 5 ms-20 ms which is the time integrity constant of the neuron. High Voltage gradients lead to great neuronal excitability and possibly suprathreshold depolarization. Secondly, phasic effects of stimulation are caused by the successive on/off nature of the pulsatile currents which subsequently attribute to the opening and closing Ca2+ or Na+ channels which is unlike tDCS. This leads to a gradual increase in the firing of action potentials in the targeted region of interest. A novel form of tPCS is being developed named Amplitude-Modulated tPCS (am-tPCS) where the polarity of the current being delivered between the two electrodes is repeatedly reversed over time from positive to negative and back. Four key hypotheses for amplitude modulation of stimulation are
• Increasing fractional anisotropy in a region of interest by eliciting action potential bidirectionality due to polarity reversal of stimulation,
• Reducing the ionic build-up underneath anodal electrode by reversing the direction of current flow and subsequent net ionic movement,
• Inducing entrainment upon neuronal ensembles through the coupling of the endogenous frequency with the amplitude modulated frequency, and
• Increasing functional connectivity between two cortical regions with functional connectivity due to the illicitation of synchronous firing of regions.
Descriptions of the effects of am-tPCS are sparse in literature and nonexistent in the treatment of neurodegenerative disorders. The purpose of this study was is to investigate if am-tPCS on the motor cortex can improve Parkinson’s symptoms measured through UPDRS and video analysis. Subject had the four tPCS electrodes positioned on C3+C4 and on F1+F2. A total of n=1 subject will undergo tPCS intervention, 5 days a week, for 1 week (5 sessions total), 20 minutes each session.
Inclusion criteria in case study
• Subjects ages 30-80.
• A diagnosis of Stage IV/V parkinson’s disease Hoehn and Yahr scale.
Exclusion criteria in case study
• Have lost their ability to consent to their own treatment
• Loss of autonomy
• Drug-induced Parkinsonism
• Patients who cannot walk independently or wheelchair-bound
• Individuals who do not have a regular caregiver (A caregiver is defined as someone who is with the subject everyday)
• Any contraindication to tCS (e.g., skin disease or treatment causing irritation)
• Any neuromodulation therapy (e.g., ECT, transcranial magnetic stimulation (rTMS), tDCS) within the last month
• Current or past (within the last 1-month) use of anticonvulsants, lithium, psychostimulants, dexamphetamines, carbamazepine, current use of decongestants or other medications, including sleeping aids, previously shown to interfere with cortical excitability
• History of Seizure or previous diagnosis of any seizure disorder
Case subject age
76 years old.
Case subject gender
Female.
Complete diagnosis list
• Grade II Arterial Hypertension.
• Parkinson's Stage IV.
• Renal insufficiency.
• Uncomplicated malaria.
Patient history of parkinson’s disease
Right upper limb tremors began 20 years ago with tremors moving to the left upper limb two years later. The tremor in the hand and toes began a few years after the left upper limb tremors. Furthermore, the patient has a history of impaired short-term memory for recent and immediate events for the past three years as a possible side effect of the progression PD degeneration. The patient has reported episodes of peripheral facial paralysis and she had experienced unconsciousness and general physical asthenia in the past. The patient is conscious now
Patient complete medical history
• Arterial hypertension documented for 22 years under treatment consisting of:
i. Carvedilol S/1 × 1/2/day, Enalapril Co 20 mg s/1 Co/day,
ii. Furosemide Co 40 mg S/1 × 1/2 Co/day
• History of stroke with peripheral facial paralysis for 20 years.
Physical examination
• General condition altered by physical asthenia and unconsciousness.
• Vital signs: BP: 169/83 mmHg HR 81 bpm SpO2: 99%.
• Well colored patient, the bulbar conjunctivae are anicteric.
• Trembling of both hands preventing her from holding objects with both hands and tremors of the toes preventing her from walking.
• Discreet edema peri-malleolar and taking the pit.
Current treatment protocol
• R/ Ceftriaxone S/2×1g/d
• R/ Hygieno-dietary measures
• R/ Furosemide Co 40 mg s/2 × 40mg/d
• R/ Aldactone co 25 mg s/2 × 25 mg/d
• R/ paracetamol co 500mg s/2 × 1g/d
• R/ Relax s/2 ×1 sachet/d
• R/ Malaxin plus s/2 ×1 tab/d
• R/Physiotherapy that people with ASD struggle with explicit VPT.
A 76-year-old female patient is investigating am-tPCS for her right/left upper limb resting tremors that were first observed 20 years ago. Subject underwent proper screening and signed treatment consent as well as video release forms prior to being initiated into the study. The Movement Disorders Society-Unified Parkinson's Disease Rating Scale (MDSUPDRS) was administered on the first and last visit after five sessions of stimulation over one wees. Cognitive and gait assessments were carried out before on the first (pre intervention) and last visits (post intervention). A visual based estimation of the MD-UPDRS gait scores was by physician recorded videos after the subject signing of the video-release forms at the beginning and end of the week, before and after the stimulation respectively. Videos were taken of the UPDRS gait assessment as well as a 30 second video of the resting hand. Videos were analyzed using custom software to generate severity estimations of the gait and amplitude/frequency time series of the resting tremor.
Duration
5 days.
Unified parkinson’s disease rating scale
The Unified Parkinson's Disease Rating Scale (UPDRS), is the most used scale in the clinical study of PD and it follows the longitudinal course of PD. The UPDRS is made up of these sections:
• Part I: evaluation of mentation, behavior, and mood,
• Part II: self-evaluation of the activities of daily life (ADLs) including speech, swallowing, handwriting, dressing, hygiene, falling, salivating, turning in bed, walking, and cutting food,
• Part III: clinician-scored monitored motor evaluation,
• Part IV: complications of therapy,
• Part V: Hoehn and Yahr staging of severity of Parkinson's disease, and Part VI: Schwab and England ADLF scale [18].
Video analysis
A customized U LLC Video Analysis software was used to measure the resting tremor of subjects by digitizing the hand in the video and measuring the degree of changes of key nodes mapped unto the video.
The intervention of transcranial amplitude modulated pulsed current stimulation
Subject will have the four tCS electrodes positioned on C3+C4 and on F3+F4, supraorbital area of the more affected hemisphere. A total of n=1 subject will undergo tCS intervention, five days a week, for one week (five sessions). Stimulation will be applied for 20 minutes with real stimulation waveform parameters of 500 ms Pulse-Interval (PI), 50 ms Inter Pulse Interval (IPI). The first 5 minutes of stimulation will be between F3+F4 where stimulation will be Amplitude Modulated (AM) at 0.45 Hz (2.2 sec.) and the last 15 minutes of stimulation where stimulation will alternate between C3-F4 and C4-F3 at montage modulation of 0.1 Hz (10 sec) (Figure 1). AM refers to the frequency polarity of the amplitude being reversed. The four key hypotheses for amplitude modulation of stimulation are:
Figure 1: Amplitude modulated transcranial pulsed current stimulation.
• Increasing fractional anisotropy in a region of interest by eliciting action potential bidirectional due to polarity reversal of stimulation.
• Reducing the ionic build-up underneath anodal electrode by reversing the direction of current flow and subsequent net ionic movement.
• Inducing entrainment upon neuronal ensembles through the coupling of the endogenous frequency with the amplitude modulated frequency.
• Increasing functional connectivity between two cortical regions with functional connectivity due to the elicitation of synchronous firing of regions.
Corticospinal tract
There is some evidence that suggests Corticospinal Tract (CST) impairments do occur in PD (Figure 2). PD degeneration is not strictly confined to the nigrostriatal dopaminergic pathway and increased white matter Fractional Anisotropy (FA) is observed in the cortico-basal ganglia-thalamo-cortical neural network. This increased FA in the motor tract in PD suggests compensatory neuroplasticity or selective neurodegeneration. Another piece of evidence suggesting the corticospinal tract is abnormal in PD compared to healthy controls is through Transcranial Magnetic Stimulation (TMS) testing of the Central Motor Conduction Time (CMCT) which represents the maximum conduction velocity of corticospinal axons. In patients with PD, a significant reduction in CMCT was observed compared to healthy controls suggesting action potential propagation impairment in the CST because of possible maladaptive neuroplasticity. These findings from neuroimaging and electrophysiological data suggest functional and structural changes of CST in PD patients [19-20].
Figure 2:Transcranial pulsed-current stimulation with parkinson’s disease.
A 76-year-old female patient with diagnosed Stage IV Parkinson's Disease was recruited in the case study using am-tPCS over her motor cortex, along the corticospinal tract, for five days, 20 minutes each day. Video analysis showed a significant reduction in a tremor from Day 1 to Day 5 from the average tremor amplitude on day 5 being 12 cm and ending tremor average of 2.5 cm at Day 5. This result is an estimated 80.0% tremor reduction in resting hand tremor amplitude over the course of five days of amtPCS treatment. On the UPDRS Day one, the Non-Motor Symptoms of Daily Living, Motor Experiences of Daily Living, and Motor Examinations scores was 40, 22, and 6 respectively with a total score of 68 (Figure 3). On Day five, the scores were 9, 7, and 2 respectively with a total score of 18 with a total UPDRS Score reduction of 73.5% from baseline. The non-adverse events reported from am-tPCS were skin tingling.
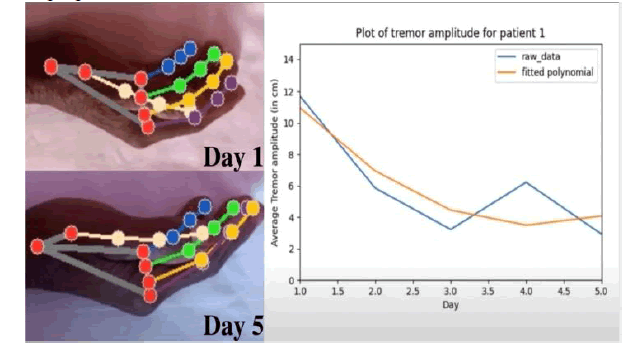
Figure 3: Plot of tremor amplitude.
This is the first study to date testing out the novel concept of amplitudemodulated stimulation and the first study using am-tPCS in the Parkinson’s Disease case. Overall, an estimated 80.0% tremor reduction was observed via video analysis, and a reduction of 73.5% was observed in UPDRS. This study suggests that am-tPCS is safe and efficacious in the PD population in comparison to other stimulation waveforms and further research is needed to map out the safety and efficacy profiles of am-tPCS in PD and other neurodegenerative disorders.
The authors have no conflicts of interest to declare.
U: The Mind Company, Cleveland, Ohio.
Citation: Abouelsoud, M., et al. Case Report of Transcranial Pulsed-Current Stimulation with Parkinson’s Disease . 2023, 09 (03), 001-004
Received: 09-Mar-2023, Manuscript No. cep-23-91151; Editor assigned: 11-Mar-2023, Pre QC No. cep-23-91151 (PQ); Reviewed: 16-Mar-2023, QC No. cep-23-91151 (Q); Revised: 18-Mar-2023, Manuscript No. cep-23-91151(R); Published: 30-Mar-2023, DOI: 10.35248/ 2471-2701.22.9(3).337
Copyright: : ©2023 Abouelsoud, M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : U: The Mind Company, Cleveland, Ohio