Review Article - (2024) Volume 15, Issue 2
Background: It is believed that the main pathological changes in Alzheimer's disease are abnormal deposition of β-amyloid (Aβ) protein and phosphorylation of tau in the brain, and various in vitro and in vivo experiments have shown that aquaporin 4 (AQP 4) may be responsible for Aβ metabolism and clearance in Alzheimer's disease.
Objective: To provide a new direction for the diagnosis and treatment of Alzheimer's disease by characterizing the structure and function of AQP 4 and the function and possible mechanism of AQP 4 in the clearance of Aβ in Alzheimer's disease.
Conclusion: Strengthening of the regulatory effect of Aqp 4 on lymphatic function in the brain has an important effect on the development, progression and outcome of Alzheimer's disease. Aqp 4 agonists or agents that induce AQP 4 opening are expected to become novel treatments for Alzheimer's disease.
AQP4 • Alzheimer's disease • Astrocytes • Glial lymphatic system • Cognition
AQP4, the most abundantly expressed aquaporin in the brain, is highly expressed in astrocyte foot processes and around blood vessels and plays an important role in lymphatic circulation, as shown in Figure 1[1]. AQP4 is a tetramer composed of 4 subunits with different activities. The amino and carboxyl termini of AQP4 are located intracellularly, the sequences of the front and back parts of the molecule are similar, and the structure of the molecule is symmetric [2]. AQP4 is involved in water transport and plays an important role in stellate cell migration, as well as in inflammatory processes during the immune response [3]. Astrogliosis is an early pathological change in Alzheimer's disease that precedes Aβ deposition [4]. Disruption of the polar distribution of AQP4 in the brains of patients with Alzheimer's disease leads to a decline in the clearance rate of the glymphatic system, the abnormal accumulation of harmful substances such as Aβ and Tau protein, the formation of amyloid plaques in the brain parenchyma, and the development of cognitive dysfunction [5]. As more researchers have focused on the role of AQP4 in the progression of Alzheimer's disease pathology, they have hypothesized that AQP4 regulates the glymphatic system in addition to serving as a channel for small-molecule drug transport to increase the proportion of drugs that enters the Central Nervous System (CNS) [6], and that AQP4 agonists may modulate the clearance of Aβ and other metabolites to delay the progression of cognitive dysfunction and neurodegenerative diseases such as Alzheimer's disease.
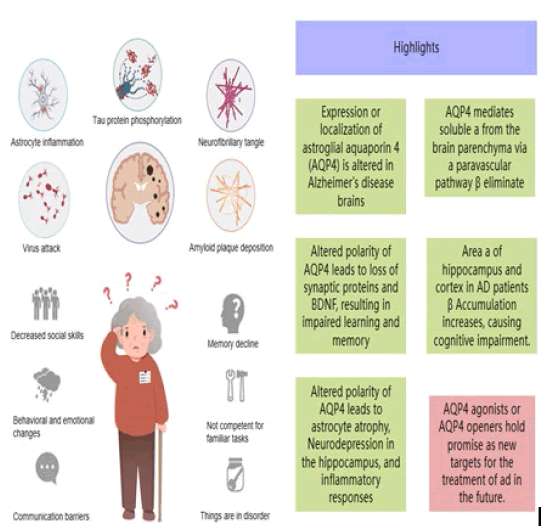
Figure 1: Alzheimer's disease causes a variety of neurodegeneration and physical changes.
Overview of AQP4
Structural distribution and physiological roles of AQP4: AQP4 is a major subtype of AQP in the adult brain and is the main water channel in the brain. It is mainly expressed in astrocytes, especially in peripheral vessels of the CNS and near the bloodâ??brain barrier [7]. The polarized distribution of AQP4, which is one of its essential features, is also required for it to exert its physiological functions in regulating body fluid exchange [8]. AQP4 has been implicated in brain inflammation initiation, glymphatic fluid clearance, maintenance of synaptic plasticity, learning and memory formation, and potassium homeostasis [9]. Furthermore, the glial water channel AQP4 is the most abundant water channel in the brain, spinal cord, and optic nerve and plays a major role in maintaining water homeostasis in the CNS, controlling the perivascular exchange of Cerebrospinal Fluid (CSF) and Interstitial Fluid (ISF) [10]. AQP4 influences the clearance of the pathological protein Aβ by mediating the glymphatic system, thereby reducing the concentration of Aβ in the extracellular space and reducing Aβ toxicity [11]. Investigating how the clearance of Aβ is regulated by changes in the polar distribution or localization of AQP4 will have profound implications for the diagnosis and treatment of Alzheimer's disease, a diagram of this phenomenon is presented in Figure 2 [12].
Figure 2: The upper left panel shows CSF-ISF exchange in healthy people, and the upper right panel shows CSF-ISF exchange in patients with Alzheimer's disease. AÃÂ?², Tau protein deposition, neurofibrillary tangle formation, cell degeneration and necrosis, and decreased CSF-ISF exchange occur in the brains of Alzheimer's disease patients.
How AQP4 affects the progression of Alzheimer’s disease
AQP4 affects astrocytes: AQP4 is mainly expressed on the end feet of astrocytes that compose the bloodâ??brain barrier, regulating the water balance in the brain, and a small amount is distributed on ependymal cells [12]. AQP4 is transported to water molecules through a membrane potential-independent and non-saturating mechanism. Therefore, it can increase the permeability of the cell membrane to water by several to tens of times [13]. This property allows AQP4 to play an important role in astrocyte migration processes. AQP4 is the most abundant aquaporin in the CNS, and it is highly polarized and found adjacent to brain micro vessels or astrocyte processes [14]. AQP4 has regulatory effects on various astrocyte functions and astrocyte-related processes, such as astrocyte activation and migration, the inflammatory response, and adult neurogenesis and neurotransmission [15, 16]. AQP4 mediates intracellular and intercellular fluid transport in the CNS and CSF transport in many neurodegenerative diseases, such as brain microvascular disease, Alzheimer's disease, and Traumatic Brain Injury (TBI), and research has found that the expression of AQP4 is closely related to the clearance function of the lymphatic system. AQP4 can regulate water transport and signal transduction, participate in the secretion of neurotrophic factors, and promote the clearance of metabolites and neurotoxic substances [17]. Astrocytes are the most widely distributed neural cells within the mammalian CNS, accounting for approximately half of the brain volume. Each astrocyte can interact with 2,000,000 synapses to exert a variety of complex physiological functions [18]. For example, astrocytes maintain environmental homeostasis in the brain, and regulate cerebral blood flow, energy metabolism, neurotransmission, synaptic plasticity, neural development and regeneration. AQP4 can affect the progression of Alzheimer’s disease via astrocytes. In the early stages of the disease, activated astrocytes can engulf pathological neurons and some pathogenic factors, such as Aβ and PD bodies, reducing oxidative stress [17, 19]. By secreting neurotrophic factors, astrocytes maintain cellular microenvironment stability, which is beneficial to some extent for disease outcomes. However, as CNS diseases such as Alzheimer's disease progress, astrocytes cause loss of basic function, increase reactive oxygen species levels, and secrete excess inflammatory factors, such as Interleukin-1β (IL-1β), Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α), causing damage to neurons, synapses, and microtubules and accelerating the course of the disease [20]. AQP4 deletion slows the migration of astrocyte cell bodies and favors the formation of a glial network in which microglia surround plaques, isolating the plaques from surrounding tissue and preventing further diffusion of Aβ and the progression of pathology [21]. AQP4 deficiency in APP/PS1 (Alzheimer’s disease) mice alleviates reactive astrogliosis at 6.5 months of age but attenuates astrocyte proliferation at 12 months of age, possibly due to inceased astrocyte atrophy caused by accumulation of Aβ. These studies suggest that the effect of AQP4 deficiency on astrocyte reactivity depends on the type and stage of neurological disease.
AQP4 is involved in the clearance of Aβ and Tau: Astrogliosis is an early pathological change in Alzheimer's disease that occurs well before the deposition of Aβ. In the early course of Alzheimer's disease, mild activation of astrocytes is conducive to the repair of degenerative nerve tissue and Aβ clearance and transport from the brain [22]; however, with the progression of the disease, Aβ load increases, Aβ accumulates, Aβ induces astrocyte atrophy, many inflammatory factors are released, Aβ clearance is blocked, and the inflammatory reaction and neuronal damage are aggravated, forming a vicious cycle [23]. There are multiple Aβ clearance mechanisms in the brain to prevent Aβ accumulation, and the most important is the bloodbrain barrier [24]. However, in Alzheimer's disease patients, the structure of the bloodâ??brain barrier is altered, bloodâ??brain barrier function is impaired, and Aβ cannot be cleared and thus accumulates in cerebral blood vessels and the brain parenchyma, resulting in the formation of neurodegenerative lesions [25]. Moreover, disruption of the bloodâ??brain barrier leads to accumulation of metabolic waste products, inducing inflammation, which further increases the risk of Alzheimer's disease [26]. A vicious cycle is formed between bloodâ??brain barrier dysfunction and Aβ deposition, leading to disease deterioration. Soluble Aβ clearance by the glymphatic system was found to be decreased by 55% in AQP4 knockout mice compared with wild-type mice, and impairment of the AQP4-mediated para-vascular pathway may account for the accumulation of Aβ [27]. The glymphatic system is also involved in the clearance of Tau. Tau protein was injected into the striatum of mice 15 min after the application of an AQP4 blocker. The results showed a 90% decrease in the clearance of Tau in the brains of AQP4 blocker-treated mice compared to those of controls [28]. The aggregation of Tau protein causes the degeneration of nerve cells and the formation of neurofibrillary tangles, which can aggravate cognitive dysfunction and persistent neuro-inflammation in Alzheimer's disease.
AQP4 affects learning and memory consolidation: Hippocampal neurogenesis plays an important role in the acquisition and storage of memory, and the main areas involved in adult neurogenesis are the subgranular zone of the hippocampal dentate gyrus and the sub-ventricular zone in the lateral outer wall of the lateral ventricles [12]. Deficiency of AQP4 and disruption of its polarity in the brains of Alzheimer's disease patients reduce adult neurogenesis and the integration of newborn neurons into learning- and memory-related neural circuits, impair object location memory, and affect the acquisition and storage of memory. AQP4-null mice have selective spatial memory impairment and obvious learning and memory dysfunction, including impairment of object location memory and consolidation memory, which involve the hippocampus; impairment of fear memory, which involves the amygdala, and dysfunction of the motivational system and information processing [29]. Astrocyte end feet, together with presynaptic and postsynaptic membranes, form a trisynaptic structure that enables astrocytes and neurons to effectively communicate and is essential for memory formation and consolidation [30]. Different astrocytes can also connect with each other through gap junctions on their foot processes, forming a vast astrocyte network, and deletion of AQP4 or alteration of its polarity affects this network. Calcium waves generated by single astrocytes can be rapidly propagated through the astrocyte network, facilitating the regulation and integration of information, and AQP4 affects learning and memory by affecting the function of astrocytes in synaptic structures and the formation and propagation of astrocyte-mediated calcium waves [15, 31]. AQP4 can also affect the phagocytic ability of astrocytes and the secretion of inflammatory factors, affect the clearance of metabolites and neurotoxic factors in the extracellular space and thus affect the progression of cognitive impairment. AQP4 also reduces adult neurogenesis by affecting the secretion of neurotransmitters and growth factors and the expression of their receptors, thereby affecting the acquisition and consolidation of learning and memory [32]. Synaptic damage in the hippocampus and cerebral cortex is the major pathological basis of cognitive damage in Alzheimer's disease [33]. Loss of AQP4 reduces synaptic protein expression in the brain and Brain-Derived Neurotrophic Factor (BDNF) production. BDNF is a key regulator of neuronal survival and synaptic plasticity and is required for long-term learning and memory [34]. Inhibiting the activation of CREB (a transcription factor that regulates BDNF expression) accelerates Aβ deposition and triggers Alzheimer's disease-associated neurodegeneration.
Animal experiments have found that cognitive dysfunction is increased in APP/PS1 mice with AQP4 knockout [35]. AQP4 loss may affect learning and memory through Glutamate Transporter 1 (GLT-1); impair Long-Term Potentiation (LTP) in the hippocampus; downregulate the expression of glutamate transporters in astrocytes; inhibit the release of glutamate, Potassium (K+) and Calcium (Ca2+) from astrocytes; impair synaptic plasticity and thus learning and memory; and even cause cognitive impairment [36].
AQP4 affects the glymphatic system: The glymphatic system is composed of CSF, the perivascular Space (VRS), AQP4 and brain ISF. Water exchange through AQP4, which is mediated by astrocyte end feet, is responsible for removing metabolites (such as lactate), abnormal proteins (such as Aβ), glucose, lipids and other nutrients and neuroactive substances from brain tissue and is important for maintaining the homeostasis of brain fluids [37]. The glymphatic system in the CNS has the function of removing metabolic waste, leading to Aβ deposition. Deficiency of Aqp4 in Alzheimer's disease reduces the inflow of CSF and the outflow of brain ISF, reducing Aβ clearance in the brain, leading to nerve endotoxin deposition, and aggravating brain tissue damage, astrocyte atrophy and degeneration [38, 39]. AQP4, the most highly expressed aquaporin in the brain, is highly expressed in astrocyte foot processes and is capable of facilitating the flow of water and some ions in the ISF. Approximately 40% to 80% of large protein molecules and solutes, respectively, are cleared by the glymphatic system. Nutrients enter the CSF through the VRS of the mucous membrane and perforating arteries and are taken up by nerve cells, and metabolic waste is transported out of the brain parenchyma through ISF [40]. Normal AQP4 structure and function are important for the outflow of ISF from the brain. After Aqp4 deletion, elevation of tissue volume and cellular edema increase ISF flow resistance, which significantly decreases the flow of ISF out of the hippocampus [41]. AQP4 is beneficial for maintaining the integrity of the bloodâ??brain barrier, and AQP4 covers almost the entire cerebrovascular barrier and CSF barrier in the mouse brain. AQP4 is typically highly polarized and densely expressed at the ends of blood vessels around the cerebral vasculature [42], and it plays a key role in regulating liquid flow. Studies have shown that AQP4 expression and polarization are dynamically regulated and essential for efficient fluid transport [43]. Animal studies have proven that AQP4 knockout or application of AQP4 inhibitors attenuates CSF influx into the brain parenchyma and reduces extracellular tracer clearance [44]. Extensive astrogliosis and loss of perivascular AQP4 polarization can occur in the brains of patients with Alzheimer's disease, especially in cortical areas, where the glymphatic circulation occurs and metabolites are deposited in the brain. Altering the localization and polar distribution of AQP4 around blood vessels to enhance glymphatic system function is a novel therapeutic strategy for Alzheimer's disease [45].
AQP4 polarization and sleep: Sleep maintains the homeostatic balance of brain metabolites by initiating the lymphatic system, para-vascular Aβ clearance is regulated by the sleep wake cycle, and loss of para-vascular AQP4 protein leads to Aβ clearance impairment and even triggers cognitive impairment [46]. A orexin dual receptor antagonist (Dora, a hypnotic agent used to treat mild to moderate sleep disorders in Alzheimer's disease) attenuates the impairment of learning and memory caused by sleep disturbance in Alzheimer's disease mice, and the mechanism is associated with the alleviation of AQP4 polarity disruption [47] and promotion of a sleep-related lymphatic system pathway that increases Aβ clearance [48]. Approximately 40% of patients with Alzheimer's disease experience sleep problems to various degree, and sleep disturbances begin to play a role in Alzheimer’s disease progression in the initial stages of the disease; therefore, AQP4-targeted treatments for sleep disturbance may be important for delaying the progression of Alzheimer's disease pathology and alleviating cognitive impairment [49]. Polarization of AQP4 is regulated by the circadian rhythm, with AQP4 perivascular polarity being the highest during the rest phase and decreasing during the active phase, and alteration of AQP4 polarity is associated with increased CSF influx and increased brain clearance [50, 51]. There is a link between lymphatic function in sleep and the maintenance of cognitive function [52]. The glymphatic system functions during sleep, and its activity is suppressed in the active state [43]. A previous study found that peri-arterial inflow and overall brain parenchymal flow in mice were higher during sleep or anesthesia than during the awake state, and the clearance rate of Aβ during sleep was more than one times higher than that during the awake state [53].
When sleep deprivation occurs, glymphatic system function is limited, and peri-arterial inflow into the brain parenchyma significantly slows, resulting in decreased deposition of Aβ in cognition-related brain regions. The lymphatic system mainly functions during sleep. The increase in the function of the lymphatic system during sleep results in improved clearance efficiency via inhibition of adrenergic activity, an increase in the interstitial space, and a subsequent increase in CSF capacity [54].
AQP4 has received increasing attention in Alzheimer's disease research. The polar distribution of AQP4 is closely related to Aβ clearance. Studying AQP4 in the brain can provide a better understanding of the pathological mechanism of Alzheimer's disease [54]. AQP4 agonists or agents that promote AQP4 opening can promote Aβ clearance from the brain interstitium, which may have important implications for preventing the development and progression and improving the outcomes of Alzheimer's disease [33]. AQP4 is important for maintaining glymphatic system function and thus promoting the flow of ISF out of the brain and represents a novel target for preserving glymphatic pathway function to maintain efficient clearance of interstitial solutes [55]. Moreover, AQP4 knockout mice show an increased seizure threshold, prolonged seizure duration, and decreased kinetics of inwardly rectifying potassium channels, indicating that AQP4 may be involved in the maintenance and alteration of brain excitability and affect learning and memory consolidation in hippocampal and cortical regions [56]. In this review, we briefly describe recent research progress related to the role of AQP4 in Alzheimer's disease [32].
We believe that as more relevant basic and clinical research is performed, our understanding of the role of AQP4 in Alzheimer’s disease will improve, ultimately benefitting patients with Alzheimer's disease.
We thank all authors who contributed data to our analyses, and the foundations and committees that supported this study and thanks for language assistance during the preparation of this manuscript. Two authors participated independently in the design, implementation, and evaluation of the article and had formal training
Articles have been scrutinized with a professional anti plagiarism literature detection system prior to publication. Article statistical methods have been approved by Shandong First Medical University Graduate School Biology. This is an open access article which, under the terms of the Creative Commons license (4.0) (attribution 1 noncommercial share identical by descent), permits others to edit, adapt, and expand on the original article for non-commercial purposes, provided that the article is read, downloaded, copied, transmitted, printed, retrieved, hyperlinked by any user, And building for indexing, to be used as input data to the software or for any other legitimate use.
Fund programs: National Natural Science Foundation of China (based on orexin-A signal transduction characteristics to explore the molecular and cellular mechanisms of sleep deprivation impairing learning and memory, 81471345), Natural Science Foundation of Shandong Province (Research on the effects of sleep disturbances on AD learning and memory and their molecular and pathological mechanisms, ZR2020MH160), Shandong University Horizontal Program (Comparative Study on Early Intervention of Sleep Disorders to Delay Alzheimer's Disease, 089/2019 horizontal).
Citation: Zhou, M., Tang, J. AQP4 Participates in Cognitive Regulation in Alzheimer's Disease. J Neuro Neurophysiol 2023, 14 (7), 001-005
Received: 28-Mar-2024, Manuscript No. JNN-24-104128; Editor assigned: 29-Mar-2024, Pre QC No. JNN-24-104128(PQ); Reviewed: 07-Apr-2024, QC No. JNN-24-104128(Q); Revised: 09-Apr-2024, Manuscript No. JNN-24-104128(R); Published: 16-Apr-2024, DOI: 10.35248/2332-2594.23.14 (6).341
Copyright: © 2024 Zhou M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.