Review Article - (2021) Volume 7, Issue 2
Infectious bursal disease (IBD) is caused by a virus that is a member of the genus AviBirnavirus of the family Birnaviridae. Although turkeys, ducks, guinea fowl and ostriches may be infected, clinical disease occurs solely in chickens. Only young birds are clinically affected. Severe acute disease of 3-6-week-old birds is associated with high mortality, but a less acute or subclinical disease is common in 0-3-week-old birds. This can cause secondary problems due to the effect of the virus on the bursa of fabricius. IBD virus (IBDV) causes lymphoid depletion of the bursa and if this occurs in the first 2 weeks of life, significant depression of the humoral antibody response may result. Two serotypes of IBDV are recognized; these are designated serotypes 1 and 2. Clinical disease has been associated with only serotype 1 and all commercial vaccines are prepared against this serotype. Very virulent strains of classical serotype 1 are now common and are causing serious disease in many countries. In Ethiopia a recent country wide study reported IBDV seropositivity rates in backyard chickens to be close to 92%. Clinical disease due to infection with the IBDV, also known as Gumboro disease, can usually diagnosed by a combination of characteristic clinical signs and post-mortem lesions. Laboratory confirmation of disease, or detection of subclinical infection, can be carried out by demonstration of a humoral immune response in unvaccinated chickens or by detecting the presence of viral antigen or viral genome in tissues. In the absence of such tests, histological examination of bursa may be helpful. Death of chickens usually starts at the 30th day of age and continues to the 5th day after infection and with falling spiking curve. Add control and prevention and your recommendations.
Bursa of fabricius • Ethiopia • Infectious bursal disease • Mortality
Infectious bursal disease (IBD), an immunosuppressive disease of young chickens, has been responsible for major economic losses in the poultry industry worldwide, particularly for the past decade. The disease affects lymphoid organs, i.e. lymphoid cells in bursa of Fabricius, resulting in lymphoid depletion and the final destruction of the bursa as the predominant feature of the pathogenesis of IBD. Chickens are highly susceptible to the virus between 3 and 6 weeks after hatching, when the bursa of Fabricius reaches maximum development. There are two serotypes of IBDV (serotypes 1 and 2). Strains of serotype 1 IBDV are pathogenic only in chickens, and are further classified as classical virulent IBDV, very virulent IBDV, antigenic variant IBDV and attenuated IBDV [1].
In the world, the poultry industry has encountered heavy economic losses associated with very virulent (vv) IBDV strains in the last several years. Variant IBDVs do not cause mortality, whereas the classical strains cause up to 20% mortality. IBDV is very stable and resistant to many disinfectants, and therefore vaccination is considered as the best way to control the disease. In the late 1980's, the emergence of very virulent IBD virus (vvIBDV) in Western Europe, changed IBD situation from mainly subclinical infection causing less than 1% mortality and satisfactorily controlled by vaccination to severe infection causing mortality up to 25% in broilers and 60% in layers. It was no longer possible to protect broilers with vaccines produced from mild vaccinal strains because it was obvious that vvIBDV could break through immunity provided by highly attenuated vaccine strains [2].
As a result, it was necessary to introduce more invasive strains in vaccine production. On the other side, it is well known that less attenuated strains may cause lesions in the bursa follicles followed by immunosuppression, particularly if 'hot' vaccines are used. Although their immunogenicity is a must, it is important for live IBD vaccine to be considerably safe [3]. Therefore, the objectives of this seminar paper are:
• To review the importance of infectious bursal disease in poultry.
• To highlight the economic importance in poultry production.
Historical background
Cosgrove 1962 reported a specific disease, (IBD) that affecting the bursa of Fabricius in chickens. The first cases were seen in area of Gumboro, United States of America (USA), which is the name derived, even if the terms 'IBD' / 'infectious bursitis' are more accurate descriptions. In the year of 1960 and 1964, the disease observed in most part of the USA and become devastating disease in Europe in the years of 1962 to 1971. With its pandemic movement from the year 1966 to 1974, the disease was reported in the southern and western Africa, Far East, Middle East, India and Australia [3].
Infectious bursal disease currently become an international issue, 95% of the 65 countries that responded to a survey conducted by the OIE, 1995 announced presence of infection, including New Zealand which had been free of disease until 1993.Only chickens develop IBD after infection by serotype 1 viruses. The age of maximum susceptibility to IBDV is between 3 and 6 weeks, which is the period of maximum bursa development, during which the acute clinical signs are observed. Infections occurring before the age of three weeks are generally subclinical and immunosuppressive. Clinical cases may be observed up to the age of fifteen to twenty weeks [4].
Cosgrove 1962 reported a specific disease, (IBD) that affecting the bursa of Fabricius in chickens. The first cases were seen in area of Gumboro, United States of America (USA), which is the name derived, even if the terms 'IBD' / 'infectious bursitis' are more accurate descriptions. In the year of 1960 and 1964, the disease observed in most part of the USA and become devastating disease in Europe in the years of 1962 to 1971.With its pandemic movement from the year 1966 to 1974, the disease was reported in the southern and western Africa, Far East, Middle East, India and Australia [3].
Etiology
Infectious bursal disease virus (IBDV) is an etiology of infectious bursal disease “Gumboro disease”, which belongs to a genus AviBirnavirus of family Birnaviridae. It is a double strand an RNA virus (dsRNA) virus and a non-enveloped, icosahedral capsid with bisegmented genome. The larger segment, A, is 3261nucleotides long and contains two open reading frames (ORF) and encodes four viral proteins designated as VP2, VP3, VP4 and VP5 and also the smaller segment B encodes only VP1 which has polymerase activity. The two viral proteins, VP2 and VP3 are structural proteins which form the viral capsid. The epitopes responsible for the induction of neutralizing and protective antibodies are located on the VP2 protein [5].
Non enveloped, single-shelled T=13 icosahedral symmetry capsid of about 70 nm in diameter, composed of 260 trimmers of VP2 that form spikes projecting radially from the capsid. The peptides derived from pre- VP2 C-terminal cleavages remain associated within virion. VP3 forms ribonucleoprotein complex with the genomic RNA. Minor amounts of VP1 are also incorporated in the virion [6] (Figure 1).
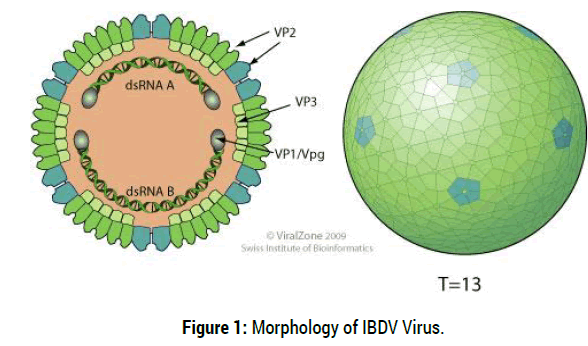
Figure 1: Morphology of IBDV Virus.
The family includes 3 genera: Aquabirnaviruswhose type species is infectious pancreatic necrosis virus (IPNV), which infects fish, mollusks, and crustaceans; AviBirnaviruswhose type species is infectious bursal disease virus (IBDV), which infects birds; and Entomobirnavirus whose type species is Drosophila X virus (DXV), which infects insects. IBDV has two serotypes of the virus. IBD virus serotype 1 and IBD virus serotype 2. IBD virus serotype 1 is an important pathogen of chickens. Serotype 2 viruses are immunologically distinct from serotype 1 viruses since vaccination with serotype 2 viruses did not confer protection against serotype 1 [7].
Physical and chemical nature of the virus: The virus is non-enveloped and quite resistant to physical and chemical agents, resistant to: pH conditions of 2–11, but it is inactivated at pH 12 due to this ability of stability and hardiness, it persists in poultry premises even after thorough cleaning and disinfection for up to 4 weeks in the bone marrow of infected chickens. The virus has been shown to remain infectious for 122 days in a chicken house, and for 52 days in feed, water and faeces [8].
Antigenic variation of IBDV strains: Historically, mutations in the IBDV genome have impacted antibody recognition and led to variations in antigenicity, immunogenicity, virulence, and tropism of circulating infectious bursal disease virus (IBDV) strains [9]. Therefore, continuous surveillance, along with rapid identification and characterization of new IBDV isolates and comparison with previously described viruses is of vital importance. The molecular basis for these emerging antigenic differences was traced to antigenic domains of the VP2 protein of IBDV. The viral capsid protein, VP2, is the major host protective immunogens, as it is the only viral protein responsible for the induction of neutralizing antibodies and for serotype specificity [10].
In Ethiopia a recent country wide study reported IBDV seropositivity rates in backyard chickens to be close to 92% [11] and IBDV isolates appear clonal and are very virulent. How the vvIBDV strains evolved in Ethiopia remains unclear. Literature suggests that international trade of live poultry and poultry products may facilitate the global spread of IBDV. Almost all acute disease outbreaks in backyard chickens in developing countries remain undiagnosed. VVIBDV isolates from wild birds and backyard chickens were shown to be highly pathogenic for SPF chickens under experimental conditions and maintain virulence marker AA residues across their VP2 and VP1 genes [12].
Epidemiology
Host range: Clinical disease occurs solely in chickens but Turkeys, ducks, and ostriches can be naturally and experimentally infected with IBDV serotypes I and II, as evidenced by serological response and isolation; however, the infections are apathogenic. Several other avian species including rooks, wild pheasants, crows, gulls, and falcons, were reported to be susceptible to infection or to possess antibodies against IBDV [13].
Serotype I viruses affect every breed of chicken, but the most severe clinical signs and lesions and the highest mortality rate have been observed in white leghorns. In fully susceptible flocks, mortality associated with classic strain infections may range from 1-60%, with high morbidity of up to100% In contrast, vvIBDV strains cause mortality of 50-60% in laying hens, 25-30% in broilers, and 90-100% in susceptible SPF leghorns [2]. According to reports the induction of a high mortality rate after IBDV infection of susceptible chickens with virulent strains correlated with the ability of the bird to mount a rapid systemic cytokine-mediated immune response, which may lead to a shock-like syndrome followed by death [14].
Transmission: Infected birds excrete virus in their dropping at least for 14 days. It is excreted in the faeces and then contaminates water, feed and litter, where it persists and from where it commonly spreads. The most common mode of infection is through the oral route, Conjuctival and respiratory routes may also be involved but the virus is highly contagious so that then disease is transmitted by direct contact with excreting subjects, or by indirect contact with any inanimate or animate (farm staff, animals) contaminated vectors between infected and susceptible flocks [15]. The high tenacity of the virus and its resistance to several disinfections and virucidal procedures may contribute to the rapid distribution of the virus. IBDV may spread through contaminated equipment [16]. There is no evidence to suggest that IBDV is spread via transovarial transmission. No specific vectors or reservoirs of IBDV have been established, but the virus has been isolated from mosquito’s (Aedesvexans), rats, and lesser mealworms (Alphitobiusdiaperinus). Viable vvIBD virus was recovered after 2 days from the faeces of a dog that had been fed tissues from experimentally infected chickens, indicating that dogs may act as mechanical vectors for the virus [17].
Morbidity and mortality: Infectious bursal disease is extremely contagious and in infected flocks, morbidity is high, with up to 100% serological conversion, after infection, whilst mortality is variable. Until 1987, the field strains isolated was of low virulence and caused only 1% to 2% of specific mortality. However, since 1987 an increase in specific mortality has been reported in different parts of the world. In the USA, new strains responsible for up to 5% of specific mortality were described. At the same time, in Europe, Africa and subsequently in Japan, high mortality rates of 50% to 60% in laying hens and 25% to 30% in broilers were observed. These hyper virulent field strains caused up to 100% mortality in specific pathogen free (SPF) chickens [18].
Pathogenesis
Following host entry via oral ingestion or inhalation, IBDV may bind to host cell proteins such as N-glycosylated polypeptide(s) expressed on the cell membrane of immature IgM+ B-cells during viral entry process. Due to its short incubation periods which range from 2 to 3 days a pore forming peptide of the virus (pep46), which is associated with the outer capsid of the IBDV particle, may facilitate viral entry into the cytoplasm of infected cells. A lipid draft mediated endocytic mechanism was suggested based on the results of an in vitro study to support entry of attenuated IBDV to the cells [19].
IBDV initiates infection and replication in lymphocytes and macrophages of the gut-associated lymphoid tissues (GALT). This stage of viral replication marks the primary viraemia. Infected macrophages transport the virus to the bursa of Fabricius (BF), the prime target organ for extensive IBDV replication in the cytoplasm of intra bursal IgM+ B-cells. After 16 hr post-infection a second viraemia occurs leading to disease and death or the virus destroys the lymphoid follicles in the bursa of Fabricius as well as the circulating B-cells in the secondary lymphoid tissues such as GALT (gut-associated lymphoid tissue), CALT (conjunctiva), BALT (Bronchial), and caecal tonsils. Virus dissemination to other lymphoid organs such as to the thymus, bone marrow, spleen, payer’s patches, cecal tonsils, and Harderian glands may take place mainly during vvIBDV infection of susceptible chickens. As early as 48 hr pi, IBDV infection induces prominent inflammation in the BF. By day 3 to 4 pi all bursal IgM+ B-cells are infected and show cytolytic changes. Clinical signs and death may result from the acute phase (710 days) of IBD [20].
Virus replication during the acute lytic phase results in a dramatic reduction in circulating IgM+ cells and a prolonged suppression of the primary antibody response. Acute disease and death is due to the necrotizing effect of these viruses on the host tissues. If the bird survives and recovers from this phase of the disease, it remains immunocompromised that inhibits protective responses of widely used vaccines against other pathogens and renders chickens susceptible to opportunisc infections. The virus preferentially affects actively proliferating and differentiating B- lymphocyts, which leads to an age-dependent immunosuppression mainly chicks infected less than one-week of age suffer severe and they may lose the entire bursal B-cells, permanent B-cell immunosuppression which result in permanent immunologic damage. The mature and competent lymphocytes will expand as a result of stimulation by the viruswhereas the immature lymphocytes will be destroyed. The bursa is infiltrated by heterophils and undergoes hyperplasia of the reticulo endothelial cells and of the inter-follicular tissue [21].
T cells are resistant to infection with IBDV may modulate the pathogenesis by limiting viral replication in the BF during the early phase of the disease at 5 days pi, by promoting bursal tissue damage and delaying tissue recovery, possibly through the release of cytokines and their concomitant cytotoxic effects. However, IBDV infection can severely decrease the in vitro proliferative response of T cells to mitogens, indicating that cellular immune responses are also compromised. Generally, the sequellae of IBDV infections such as severity of clinical signs, organ lesions and immunosuppression correlate with the status of immunity, age and genetic background of affected chickens and with the virulence of the infecting virus strain [22].
Immuno-suppression
IBDV infection in chickens activates all branches of the immune system. However, the level of activation varies depending on the virulence of infecting strains, age, immune status and genetic background of affected chickens. The immune response can be altered by maternal antibody, and the more virulent vaccine strains can override higher levels of antibody. Progeny of parent flocks vaccinated with classical strains of IBD virus may have poor maternal immunity against strains of the virus [23].
A high level of maternal antibodies will protect most young chickens against challenge by vvIBD virus for up to 3 weeks after hatching. This is borne out by the excellent passive protection provided by maternal antibodies against immunosuppression, bursal lesions, or mortality. The half-life of the passive antibodies varies between depending on breeds, three days (for broilers) and five days (for laying hens). Thus, if the antibody titre of a chick at hatch is known, then the time of maximum flock susceptibility to the wild or vaccinal virus can be determined. This information is very important when establishing the timing of vaccination programmes [10].
Pathology and lesion
Even although IBD affected different lymphoid organs the principal target of the virus is the bursa of Fabricius which is the reservoir of B lymphocytes in birds. The mature and competent lymphocytes will expand as a result of stimulation by the virus whereas the immature lymphocytes will be destroyed. Macroscopic lesions are observed principally in the bursa which presents all stagesof inflammation following acute infection [2].
Autopsies performed on birds that died during the acute phase (three to four days following infection) the bursa reveal initiallyhypertrophic, edematous and hemorrhagic and its color turns from white to cream and ayellow transudate covers its serosa early in infection (Figure A and B). The most severe cases arecharacterized by a major infection of the mucous membrane and a serous transudate, giving thebursal surface a yellowish colour and often accompanied by petechiae and haemorrhages. By thefifth day, the bursa reverts to normal size and by the eighth day becomes atrophied to less than athird of the normal size. Moreover, in the acute form of the disease caused by hyper virulentstrains, macroscopic lesions may also be observed in other lymphoid organs (thymus, spleen, caecal tonsils, Harderian glands, and Payer’s patches) [20].
On postmortem examination the affected animals have hypertrophic and whitish kidneys containing deposits of urate crystals (Figure C) and cell debris, severely dehydrated carcasses, oftendarkened pectoral muscles with many petechiaehaemorrhages masses in the thigh and pectoral muscles may be present (Figure D) and are frequently observed, probably due to a coagulation disorder. Mucus may also be present within the intestines. Liver appears pale, bile stained and grey foci may also be present on an enlarged spleen [24].
Henry has developed a system for evaluating microscopic lesions of the affected organs, with a score ranging from one to five according to lesion severity. The criteria for scoring lesions in the thymus were: 1- equaled no change; 2- cortex had a few empty spaces, pronounced hyperemia with some heterophil infiltration, especially in the medulla; 3- cortex had an increase in the number of empty spaces and increased heterophil infiltration, and the cortex and medulla had decreased hyperemia; 4- cortex had numerous round aggregations (12 to 16 mu in diameter) of cell debris and pyknotic nuclei, a definite decrease in the lymphocyte density in the cortex, and decreased hyperemia in cortex and medulla; 5- Bursa microscopically revealed complete loss of architecture. There was no intact lymphoid follicle and the entire area was filled up by fibrous tissue. The lining epithelium was highly corrugated. This lesion scoring system was useful in determining the severity of IBD in different-aged progeny from IBD-immune and non immune dams. The B lymphocytes are destroyed in the follicles of the bursa as well as in the germinal centers and the perivascular cuff of the spleen. The bursa is infiltrated by heterophils and undergoes hyperplasia of the reticuloendothelial cells and of the intermolecular tissue. As the disease evolves, the surface epithelium disappears and cystic cavities develop in the follicles. Severe panleukopenia is also observed and these microscopic lesions are exacerbated in the acute forms of the disease [25] (Figure 2).
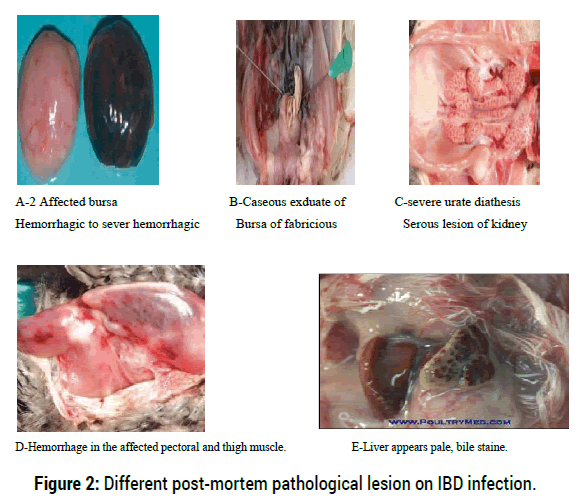
Figure 2: Different post-mortem pathological lesion on IBD infection.
Clinical sign
Infectious bursal disease virus infection severity of clinical signs and immunosuppression correlate with the status of immunity, age and genetic background of affected chickens and with the virulence of the infecting virus strain. Chickens infected between 3 and 6 weeks of age develop the most severe clinical signs of IBD. Susceptible chickens exposed to vvIBDV and classical virulent strains show a sudden onset of clinical disease within2-3 days of exposure, characterized by severe depression and ruffled feathers [26].
Chickens younger than 2 weeks of age and birds older than 6 weeks rarely develop clinical signs. Virus excretion can begin as early as 24 hours after infection. Mortality will peak and recede usually in a period of 5-7 days. Accompanying symptoms include the disease has been described worldwide as acute onset of depression, trembling, white watery diarrhoea, ruffled feathers, severe prostration, vent picking, vent feathers soiled with urates, anorexia, dehydration, and elevated water consumption [27].
Diagnosis
Embryo inoculation: The inoculation of bursal homogenates from IBDV infected chickens per the chorioallantoic membrane of 9-10 days old embryonated SPF (Specific-pathogen-free) chicken eggs is the most sensitive diagnostic method for virus isolation. The most sensitive route of inoculation is the CAM; the yolk sac route is also practicable [27]. It is important especially for Wild-type IBDV, usually not r-eplicating in conventional cell culture, can also be regenerated by the reverse genetics approach, but can grow in embryonated chicken eggs [28]. Some strains grow well in embryos but are notreadily adapted to grow in CEF (Chicken embryo fibroblasts) or CEK (Chicken embryo kidney). Variant viruses however, do not kill the embryos but cause embryostunting, discoloration, splenomegaly and hepatic necrosis [8].
Serological identification: Serological tests such as AGID, ELISA, and VNT for detecting antibodies are used for monitoring vaccine responses and might be additional information for diagnosis of infection of unvaccinated flocks. The enzyme linked immune sorbent assay (ELISA) is the most commonly used test for the detection and quantification of IBDV antibodies to check response to vaccination, natural field exposure and decay of maternal antibody titer. It is economical, simple, and quick tests a large number of samples at the same time and is adaptive to automation to computer software [8].Viral antigens can be demonstrated by the agar-gel precipitin assay or by the antigen capture enzyme-linked immunosorbent assay (AC-ELISA). The VN titers accurately correlate with protection of chickens against IBDV [29]. Differentiation of classic and variant strains has been made by using ELISA and monoclonal antibodies. However, these methods may not be as rapid and sensitive as molecular methods [30].
Identification by molecular method: The classical methods for molecular characterization and differentiation of IBDV field isolates include reverse transcriptase polymerase chain reaction (RT-PCR) and restriction fragment length polymorphism (RFLP), nucleotide sequence analysis, and quantitative real time RT-PCR (qRT-PCR) [31]. Nowadays, reverse transcription-polymerase chain reaction (RTPCR) is a molecular tool frequently applied in IBDV diagnosis. RT-PCR in combination with restriction enzyme analysis allows the rapid identification of vvIBDV. Nucleotide sequencing of RT-PCR products is widely used for further characterization of IBDV strains [32]. The VP2 gene of IBDV contains variable region which suggests the potential of this region for differentiation of IBDV strains. RT-PCR followed by digestion with multiple restriction enzymes or RFLP and nucleotide sequencing of VP2 gene have been used for differentiation of IBDV strains. The molecular differentiation of IBDV strains using VP2 has been improved by use of labeledprobes in real-time RT-PCR [33]. In recent years detection of nucleotide variation has been facilitated by application of melt curve analysis. A TaqManqRT-PCR and melting curve analysis can be used to trace mutations in the hVP2 region this method allows comparing sequences between field and vaccinal strains. It determines a single nucleotide polymorphism in VP2. Genetic typing according VP2 sequence of IBDV has been widely used as a means of tracing the spread of IBDV and virulence change [34].
Differential diagnosis: The clinical diagnosis of the acute forms of IBD is based on disease evolution of a mortality peak followed by recovery in five to seven days and relies on the observation of the symptoms and post-mortem examination of the pathognomonic lesions, in particular of the bursa of Fabricius. The diseases like avian coccidiosis, Newcastle disease in some visceral forms, stunting syndrome, mycotoxicoses, chicken infectious anemia and nephropathogenic forms of infectious bronchitis are the differential diagnosis for IBD. In all acute cases, the presence of bursal lesions allows for a diagnosis of IBD. In subclinical cases, an atrophy of the bursa may be confused with other diseases such as Marek's disease or infectious anemia. A histological examination of the bursa will allow differentiation between these diseases [8].
Treatment, prevention and control options
Treatment: No therapeutic treatment has been found to have an effect on the course of the viral infection; however birds may be helped with drugs to treat symptoms so as to control secondary agents and the effects of immunosuppression [2].
Prevention and control options
Management and hygiene procedure: Infectious bursal disease virus is both highly contagious and very resistant to inactivation which accounts for its persistent survival on poultry farms, despite disinfection so that it requires strict hygienic and managemental practice. Therefore, even with strict bio-security programs (e.g. ‘down time’ between broods, all-in/all-out production, cleaning and disinfection of the premises and equipment) is vital for prevention of IBDV infection but also vaccination is especially important to reduce the incidence and impact of IBD in the poultry industry [35].
Vaccine and vaccination: Immunization of chickens with high quality vaccines is the primary method of control of many poultry infectious diseases; However IBDV is resistant to a large variety of disinfectants and is environmentally very stable but mainly controlled by vaccination [36] with a proper vaccination schedule. Rational vaccination schedules and strict biosecurity measures were indicated in many reports as essential tools for the control of IBD [37].
Vaccines and vaccinationprogrammes vary widely, depending on several local factors (e.g. type of production, level ofbiosecurity, local pattern of disease, status of maternally derived antibodies (MDAbs), vaccines available, costs and potential losses). Many previous studies proved the role of the MDAbs in protection against IBDV in chicks. In vivo cross-protection studies, vaccination-challenge studies, and progeny challenge studies are frequently performed forassessment of IBDV vaccine efficacy and to determine the pathogenicity and antigenic phenotypes of IBDV strains [38].
More recently, an IBDV reverse genetics system was implemented to introduce selected amino acid changes into the VP2 encoding region of the classic IBDV strain D78 in order to assess antigenic determinants of IBDV [39]. This process combined with nucleotide and amino acid sequencing and MAb reactivity patterns may provide a more comprehensive analysis of IBDV strains for better diagnosis and vaccination program design.Traditionally, breeder flocks are hyper immunized by priming with live vaccines and boostingwith killed vaccine prior to laying in order to confer high titers of MAb to their progeny [40] and applied in some countries. This passive immunity protects chicks against early immunosuppressive infections for 1 to 3 weeks; however, protection may be extended to 4 or 5 weeks by boosting the immunity in breeders with oil-adjuvanted vaccines [20]. Serological monitoring of the antibody level in a breeder flock or its progeny can aid in determining the right time to vaccinate [27].
According to literature [35] oral, nasal or ocular mild vaccines were effective only in immunizing chicks that had passively acquired neutralizing antibody titers lower than 100. Two types of vaccine are mostly available for the control of IBD. These are live attenuated vaccines and killed vaccine. Live vaccines are produced from classical and variant IBDV strains by passing these viruses in tissue cultures or embryonated chicken eggs. Several liveattenuated virus vaccines that differ according to their virulence and antigenic characteristics are available commercially. With regard to virulence or residual virulence for SPF chickens, and the level of attenuation vaccine strains are classified as mild, mild intermediate, intermediate, intermediate plus, or “hot,” [4].
Live-attenuated vaccines are administered via drinking water application or nebulisation between the ages of 7 days and 2 or 3 weeks. Live vaccines are favorable for mass application through drinking water and can induce strong humoral and cellular immunity [20]. The proven reversion to virulence and their residual immunosuppressive effects are major safety concern of their extensive field applications [41].
Killed-virus vaccines in an oil adjuvant are often used to boost levels of maternal antibodies and Confer longer lasting immunity in breeder hens. The duration and uniformity of this immunity may be influenced by the concentration and antigenic specificity of the vaccine strain. These vaccines are not ideal for stimulating a primary antibody response; therefore, they tend to be most effective in chicks that have been “primed” with a live virus vaccine or naturally infected through field exposure to IBDV [20]. Currently, many oil-adjuvant vaccines contain both classic and variant IBDV strains. Killed-virus vaccines are administered by subcutaneous or intramuscular injection at sixteen to twenty weeks of age [10].
Economic importance
The economic impact of IBD in fowl is serious and influenced by strain of virus, susceptibility and breed of flock; inter current primary and secondary pathogens, and environmental and managemental factors. Clinical IBDV leads to direct losses due to high mortality, in addition, condemnation of carcasses due to skeletal muscle, thigh and pectoral muscle haemorrhages can be an important cause of economic losses [42].
Indirect losses in Gumboro disease arise due to the severe immunosuppression of broilers and egg laying hens and their increased predisposition for other diseases and vaccination failure. Thereby, as a consequence, they result delayed growth, reduced weight gain, reduced food conversion, longer fattening, lesser production values, increased mortality and lower quality of products observed [43]. The occurrence of vvIBDVs has increased the economic importance of the disease. Until 1987, the strains of the virus were of low virulence, causing less than 2% mortality, and vaccination was able to satisfactorily control the disease. However, the occurrence of vvIBDV has led to vaccination failures, and increased mortality and morbidity. In 80% of the OIE member countries, acute clinical disease due to IBDV has been reported [10].
The presence of disease may also limit opportunities in the market place, either locally or internationally, and hinder the adoption of improved technologies, improved breeds, better management systems or more efficient processing and marketing methodologies. There would be further loss of income for an extended period because of the stamping-out policy. The disruption to the flow of product and decreased production may cause job losses on farms and in service and associated industries, depending on the time it takes to bring the outbreak under control. Even a small outbreak would result in dislocation of the industry and its normal marketing patterns. An uncontrolled outbreak would markedly increase production costs because of the impact of the disease and the need for continuing control measures [44].
Infectious Bursal Disease is a newly emerging disease of chicken in Ethiopia, as described by [45] the disease has been speculated to be introduced concurrent with the increased number of commercial state and private poultry farms flourishing in the country. Research and case reports coming from various regions of the country indicated that viral diseases are posing a growing threat to the young poultry industry flourishing in the country [46]. Therefore infectious diseases like IBD are becoming real threats to chicken production. Frequent outbreaks and occurrence of new strains of infectious bursal disease became a challenge to the juvenile poultry industry in Ethiopia. Over the past few years, 25 to 75% of the deaths/losses in exotic and cross chickens have been associated with infectious bursal disease [47].
Gumboro disease was first reported in 2002 in Ethiopia at privately owned commercial poultry farm in which 45-50% mortality rate was documented and diagnosed first in commercial poultry and thereafter in a government-owned poultry multiplication center and a commercial broiler farm with serological tests. In addition to the different serological studies molecular characterization of the Ethiopian IBD virus isolates was done for the first time in 2005 from the samples collected from Kombolcha Poultry Multiplication Center, and in commercial and breeding poultry farms in Ethiopia between 2009 and 2011 [48].
In both cases the samples were processed at the National Veterinary Institute, Ethiopia, for virus isolation using chicken fibroblast cell culture, and the positive isolates were submitted to OIE-IBD Reference Laboratory, France, for further antigenic and genomic characterization, and were identified as virulent classical viruses and very virulent IBD virus. In all cases the situation of the disease at small scale commercial flocks, and back yard poultry farms indicate the disease is widely distributed in the country, More over chicken traders also suffer from huge financial losses due to IBDV mortality in chicken, particularly those who buy young aged chicken and rear them for several weeks after purchase [49].
Currently, IBD is the most important threat to poultry production in the country and widely distributed in all regions in the backyard chickens, commercial farms and poultry multiplication centers. The disease has since spread to all investigated commercial farms and multiplication centers occurring at an average outbreak rate of 3-4 farms per year. This disease has incurred considerable economic loss to the country and has been posing a challenge especially for the success of vaccines used at this time [50].
On top of this, most control strategies designed in the country do not take into consideration the local chickens, and this may lead in to the failure of most strategies. Considering the significant economic losses associated with IBDV, the development and evaluation of new generation IBDV vaccine are important to minimize the effects of these agents and design suitable preventive and control measures this tendency of growing poultry industry [51].
Poultry pathogens change their nature in response to intensified poultry production and present complex challenges to the poultry health and productivity. Very virulent IBDVs, which emerged in the late 1980s can cause mortality of up to 70% and induce severe immunosuppression. Since IBDV is ubiquitous and extremely resistant to environmental conditions, most of the efforts to control the disease are focused on vaccination programs, there is a high variation in the genetic properties between strains, these variations may play a role determining the antigenic and pathological characteristics of the viruses present in the field. In the case of RNA viruses, biological events including genetic re-assortment or recombination alter the phenotypes and genotypes of circulating viruses and compromise their genetic stability. It is clear from the present findings that IBD is one of the major poultry viral diseases causing very high mortality in chickens and prevalent in different production systems of poultry in Ethiopia and also the pathotype of the infectious bursal disease virus circulated in Ethiopia is very virulent infectious bursal disease virus (VVIBDV). How the vvIBDV strains evolved in Ethiopia remains unclear. Literature suggests that international trade of live poultry and poultry products may facilitate the global spread of IBDV. Based on above conclusion, the following recommendations are forwarded:
• Management factors like, scheduled vaccine program in backyard, proper biosecurity in semi intensive and intensive farm should be implemented to reduce the magnitude of IBDV infection in investigation area.
• Village chickens should be vaccinated against most infectious diseases including IBD.
• The persistence time of IBDV maternal antibodies (MAb) on unvaccinated SPF progeny flocks should be investigated to design an optimum vaccination schedule.
• Molecular diagnostic study should be conducted to identify the current circulating strain of IBDV in the chicken population.
• The current vaccine efficacy should be investigated.
• The Ethiopian government should set immunization schedules in chicken before and after distribute day old chickens to backyard, semi- intensive and intensive producers.
Citation: Wagari A. A Review on Infectious Bursal Disease in Poultry. Health Econ Outcome Res Open Access , 2021, 7(2): 167 (018-023).
Received: 10-Oct-2020 Published: 15-Feb-2021, DOI: 10.35248/2471-268X.21.7.167
Copyright: © 2021 Wagari A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.