Journal of Multiple Sclerosis
ISSN - 2376-0389NLM - 101654564
Research Article - (2021) Volume 8, Issue 4
Background: This Multiple Sclerosis (MS) is a Central Nervous System (CNS) disease characterized by demyelination or axonal loss encompassing several clinical subtypes—Relapsing-Remitting MS (RRMS), Secondary-Progressive MS (SPMS), and Primary-Progressive MS (PPMS). Strategies for treating progressive forms are limited, and better understanding of underlying pathophysiology is essential.
Method: Somatosensory Evoked Potentials (SEP) were analyzed for CNS conduction slowing caused by myelin impairment defined as 3 or more standard deviations, in 109 people with MS (16 with PPMS compared to 93 with RRMS and/or SPMS).
Results: People with RRMS and SPMS have a higher prevalence of CNS conduction slowing compared to PPMS (83.9% compared to 12.5% respectively, P<.001). Subgroup analysis suggests this is not due to differences in age at SEP, timing of SEP in disease course, or differences in disease severity at time of SEP.
Conclusion: CNS demyelination can be assessed by SEP and tends to be significantly less in patients with PPMS compared to RRMS or SPMS, despite similar levels of disability. This has important implications for underlying pathophysiology, suggesting that demyelination and axonal degeneration may independently contribute to disease progression.
MS Type • Somatosensory evoked potentials • Neurophysiologys
MS is considered to be an immune-mediated disease of the CNS affecting both myelin and/or axons that encompasses several clinical and pathologic subtypes—Relapsing-Remitting MS (RRMS), SPMS, and Primary-Progressive MS (PPMS) [1,2]. Approximately 85% of patients present with relapsingremitting MS, characterized by periods of acute neurological dysfunction followed by stability or improvement between these exacerbations [2]. Within 10-15 years, the majority of these patients begin to develop steady worsening, and a relative decrease in relapses, termed secondary progressive MS. Thus, SPMS appears to be a chronological worsening of RRMS [2]. In contrast, approximately 15% of MS patients do not present with relapses from onset and are diagnosed as primary progressive MS[2]. These patients are characterized by a steady progression of symptoms. The clinical demographics of these subtypes also widely differ; RRMS commonly occurs in females around age 30, whereas PPMS is seen equally in both sexes with average age of onset in the 40s [3].
Identifying mechanistic differences accounting for these varying presentations has been the subject of many studies. It is widely accepted that relapses are caused by acute episodes of inflammation within the CNS, which leads to plaques characterized by both demyelination and axonal loss. T cells and B cells are both felt to have pathogenic actions. Correspondingly, anti-inflammatory and immunomodulatory therapies (targeting either T cells or B cells) decrease the incidence of relapses. Although immunomodulatory therapies undoubtedly decrease relapses and disease progression when taken during the relapsing stage of disease, most immunomodulatory agents have been ineffective during progressive disease. The few exceptions have modest effects. Siponimod decreased the relative risk of confirmed disability progression by 21% in SPMS, but findings were not significant in those without relapses in the two years prior to study entry [4]. Ocrelizumab slows confirmed disability progression by 24% in PPMS [5]. Instead, it is felt that neurodegeneration drives disability progression in both PPMS and SPMS, supported by increasing brain atrophy in these patients, and relatively fewer gadolinium enhancing lesions [6]. However, the underlying mechanisms contributing to neurodegeneration in both PPMS and SPMS are unknown.
At present, multiple hypotheses exist regarding the etiology of neurodegeneration in progressive forms of MS. As oligodendrocytes and myelin contribute to the health of axons, it has been hypothesized that chronic demyelination may lead to progressive neuronal loss in both PPMS and SPMS [7]. However, mounting evidence also suggests that in patients with MS, neurodegeneration may occur independently from demyelination in MS [8].
The ability to study underlying disease pathogenesis in MS is difficult, as access to CNS tissue is rare, and it is difficult to evaluate pathologic changes over time. Somatosensory Evoked Potentials (SEP), which document conduction slowing (indicative of demyelination) and decreased amplitude (indicative of degeneration), may give us a better grasp of these disease subtypes and may aid in developing therapeutic strategies [9]. Historically the 1983 Poser diagnostic criteria allowed Evoked Potentials (EPs) to fulfill the “dissemination in space” criterion for the diagnosis of MS. To fulfill this criterion, EP slowing in the CNS was required since all MS was considered a “demyelinating” disease [10]. The introduction and ubiquitous application of MRI in the 1980s almost eliminated the use of EPs in MS and the physiological information it provided [11]. A few years later, McDonnell and others questioned whether all MS was a demyelinating disease, or whether there were different pathological types [12].
In this study, we analyzed SEPs collected from patients with MS, evaluating differences in underlying neurophysiology between subtypes of MS. Specifically, we compared the prevalence of CNS central conduction slowing, a well-established marker of white matter demyelination between subtypes of MS-RRMS, SPMS, and PPMS—and found that the prevalence of demyelination was significantly decreased in patients with PPMS (as compared to patients with either RRMS or SPMS) [13]. This supports the idea that symptom progression can occur independently from white matter demyelination, and suggests that white matter demyelination may not drive disability progression in patients with primary progressive MS.
Subjects
Individuals who had been diagnosed with MS and were receiving clinical MS care at the University of Washington MS Center between 1999 and 2010 were identified for this study. Of the patients seen between 1999 and 2010, a total of 121 subjects consented to at least a single SEP test in clinic throughout their care. Twelve patients were excluded from final analysis because of absent MS subtype determination, incomplete SEP testing, or a co-existing medical diagnosis that could affect SEP interpretation. Final data analysis included 109 subjects: 93 with a final subtype diagnosis of either RRMS or SPMS and 16 patients with a final diagnosis of PPMS. This study was approved by UW IRB (IRB15846).
Final subtype diagnosis
MS subtype determination is based entirely on clinical history. The subtype of each subject was designated based upon the most recent clinical assessment recorded in the UW MS Center medical record by an MS specialist. For our primary analysis, patients with a final subtype diagnosis of either RRMS or SPMS were combined into one group titled “RRMS/SPMS” (as both begin with relapsing disease and presumably the same initial pathology). For our secondary analysis patients with RRMS and SPMS were evaluated separately based on the subtype listed in the chart at the time of SEP testing. Note that some patients did not have a subtype diagnosis assigned at the time of SEP and therefore could not be subcategorized into “RRMS” or “SPMS” at that time.
Somatosensory evoked potential testing
All SEPs were performed by EP-trained technicians at the UW EMG/ SEP clinic using Caldwell Sierra Summit SEP equipment and interpreted by one of 6 different American Board of Electrodiagnostic Medicine-certified Physicians. All four limbs were studied, with stimulation over median nerves at the wrists and tibial nerves at the ankles. Signal latency was measured at several sites in the periphery and over the somatosensory cortex as previously described [14,15]. For the arms, recordings were made over Erb’s point, cervical spine at C5, and contralateral cortex at C3’ and C4’ referenced to Fz. Leg recordings were made over the popliteal space referenced to the medial knee, spine recordings were made over the L2 and T10 spinal processes referenced to the flank, and scalp recordings were made over C1’, Cz’, and C2’ referenced to Fz. Patients were categorized as having central conduction slowing if the signal entered the CNS at the expected interval but the scalp response was prolonged. If the signal was prolonged on CNS entry, delayed scalp response was moot and scalp delay was attributed to peripheral nerve slowing. Latencies were categorized as slow based on UW laboratory standards. Because of considerable variability in subject height, our laboratory has defined abnormal slowing as greater than 3 standard deviations above the average of normal healthy controls [14]. Using this standard, the following values defined slowing following median nerve stimulations: cortical N20>22.15 milliseconds (msec), cortical P25>29.95 msec, central conduction>7.3 msec. Following tibial nerve stimulations, the following values were used: cortical N33>40.7 msec, cortical P37>48.0 msec, central conduction>14.8 msec. If central conduction prolongation was noted in any CNS pathway (i.e. following either median or tibial nerve stimulation), the patient was defined as having central conduction “slowing”, consistent with a demyelinating type of MS. It is emphasized that if there was any slowing in peripheral nerve latencies accounting for delayed cortical measurements, central conduction could not be confirmed as present.
Statistics
Statistics were analyzed using SPSS software. Fisher’s exact test was used to analyze between-group differences.
Relationship between demyelination and final MS subtype diagnosis
Patients with a final subtype diagnosis of either RRMS or SPMS had a high prevalence of CNS conduction slowing. When combined into a single RRMS/SPMS group, 83.9% (78 out of 93) had evidence of central conduction slowing on SEP (Figure 1). This remained true for the separate RRMS and SPMS groups, based on their clinical diagnosis at the time of SEP. For RRMS, 50 of 57 (87.7%) demonstrated CNS conduction slowing. For SPMS, 23 of 25 (92%) demonstrated CNS nerve conduction slowing. Interestingly however, central conduction time trended towards increasing in patients with SPMS compared to RRMS, consistent with the expectation that SPMS is pathologically more advanced than RRMS. Central conduction following right-sided tibial nerve stimulation averaged 18.2 msec in patients with RRMS at the time of SEP and 24.3 msec in patients with SPMS at the time of SEP (p=.12). Although this difference was not statistically significant, the trend held true for central conduction times calculated following each of four peripheral nerve stimulation locations including the right tibial, left tibial, right median, and left median nerves. This suggests that the severity or extent of demyelination increases over the disease course as patients transition from RRMS to SPMS (Table 1).
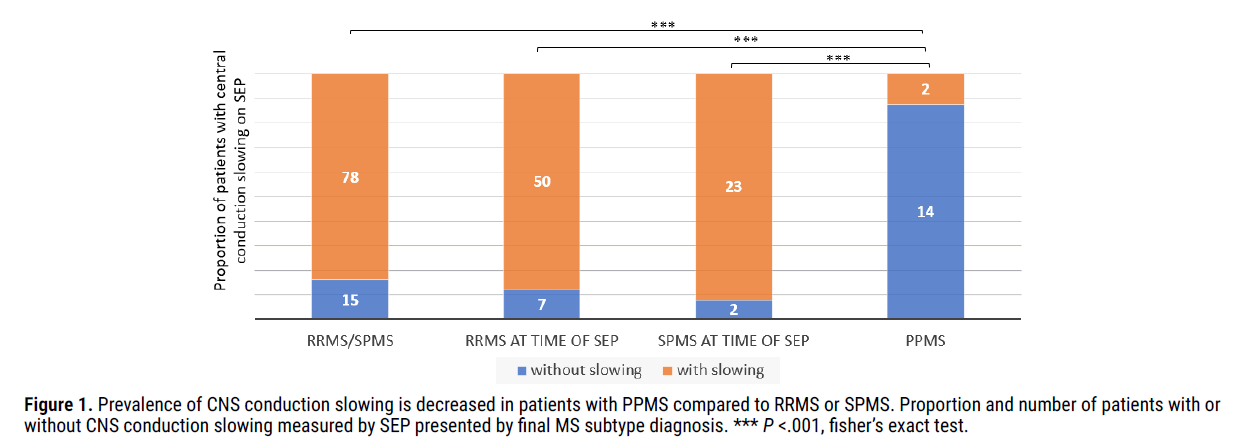
Figure 1: Prevalence of CNS conduction slowing is decreased in patients with PPMS compared to RRMS or SPMS. Proportion and number of patients with or without CNS conduction slowing measured by SEP presented by final MS subtype diagnosis. *** P <.001, fisher’s exact test.
Table 1. Central conduction differences are most pronounced following tibial nerve stimulation. Average central conduction times for median and tibial nerve stimulation compared to MS subtype at the time of SEP are shown. Central conduction time is calculated by subtracting the time for the signal to reach the lumbar or cervical spine (for tibial or median stimulation respectively) from the time for the signal to reach the somatosensory cortex. Subjects were compared against predefined Healthy control values (n=14) [14].
| Healthy controls (laboratory standard normal values) | RRMS at SEP (Average ± SEM) | SPMS at SEP (Average ± SEM) | PPMS at SEP (Average ± SEM) | |
|---|---|---|---|---|
| Right median central conduction time (msec) | <7.3 | 8.4( ± 0.4) | 10.5(1.6) | 7.2( ± 0.8) |
| Left median central conduction time (msec) | <7.3 | 8.3( ± 1.3) | 9.3(1.4) | 7.1( ± 1.3) |
| Right central conduction time (msec) | <14.8 | 18.2( ± 1.3) | 24.3(4.2) | 12.2( ± 2.1) |
| Left tibial central conduction time (msec) | <14.8 | 17.7( ± 1.3) | 22.8( ± 2.7) | 13.3( ± 2.2) |
In contrast to patients with a final diagnosis of either RRMS or SPMS, patients with a final diagnosis of PPMS had a significantly lower overall prevalence of CNS conduction slowing on SEP (12.5% in PPMS patients, 83.9% in RRMS/SPMS, P<.001), suggesting that the overall prevalence of substantial demyelination is low in patients with PPMS.
Patient characteristics differ between groups
Groups were next analyzed for underlying characteristics and potential confounding variables (Table 2). Consistent with previously published data, patients with a final diagnosis of RRMS or SPMS had a higher female predominance compared to patients with a final diagnosis of PPMS (female/ male ratio of 2.2:1 versus 1.3:1, respectively). They also had an earlier average age of initial symptom onset (32.9 years in patients with RRMS/ SPMS versus 43.1 years in patients with PPMS). Of note, average disease severity at the time of SEP was not strikingly different between patients in the RRMS/SPMS group and PPMS groups (EDSS 4.8 versus 5.1 respectively, p=.66). Although the average age at the time of SEP was slightly younger in the combined RRMS/SPMS group compared to PPMS group (46.8 years compared to 52.9 years, respectively, p=.051), the average age at the time of SEP was similar between patients with a diagnosis of SPMS and PPMS (52.9 years versus 50.5 years, respectively, p=.45). These data suggest that age or overall severity of disease does not account for the difference in prevalence of demyelination between patients with PPMS and patients with RRMS and SPMS.
Table 2. Characteristics of patients between groups. Sex, average age at time of SEP, average disease severity at time of SEP (measured by expanded disability status scale, EDSS), and average timing of SEP in years from initial MS diagnosis and years from initial symptom onset are listed for the combined group of patients with a final diagnosis of RRMS or SPMS, patients with a diagnosis of RRMS at time of SEP, patients with a diagnosis of SPMS at time of SEP, and patients with a diagnosis of PPMS at time of SEP.
| Final diagnosis of RRMS or SPMS | RRMS at time of SEP | SPMS at time of SEP | Final diagnosis of PPMS | |
|---|---|---|---|---|
| Female : Male | 2.686806 | 1.760417 | 0.754861 | 0.379861 |
| Age at first diagnosis of MS (years) | 37 | 37.6 | 37 | 48.5 |
| Age at first symptom onset (years) | 32.9 | 32.4 | 30.6 | 43.1 |
| Time from initial diagnosis to SEP (years) | 9 | 6.2 | 12.8 | 3.9 |
| Age at first SEP (years) | 46.8 | 44.4 | 50.5 | 52.9 |
| EDSS at time of SEP | 4.8 | 3.8 | 5.9 | 5.1 |
Importantly however, patients with PPMS tended to have their SEPs performed earlier in their disease course (i.e. closer to the initial time of MS diagnosis and initial symptom onset) compared to patients with RRMS or SPMS (3.9 years from initial MS diagnosis with PPMS compared to 9.0 years with RRMS or SPMS respectively, p=.03). We reasoned that this could have the potential to affect SEP results, as theoretically demyelination accumulates over time, and therefore we would expect the likelihood of detecting central conduction slowing to increase with disease duration. As such, we performed a subgroup analysis (on patients with RRMS who had their SEP performed within 3.9 years of initial MS diagnosis) to assess whether time from initial diagnosis could account for the decreased prevalence of central conduction slowing (Figure 2). Importantly, patients with RRMS who had their SEP performed within 3.9 years of their initial MS diagnosis (timing chosen to reflect average timing of SEP in PPMS group) continued to demonstrate a high incidence of central conduction slowing (17 of 19 patients, 89.5%), suggesting that timing of SEP in disease course does not account for the difference in prevalence of demyelination between patients with PPMS and patients with RRMS or SPMS.
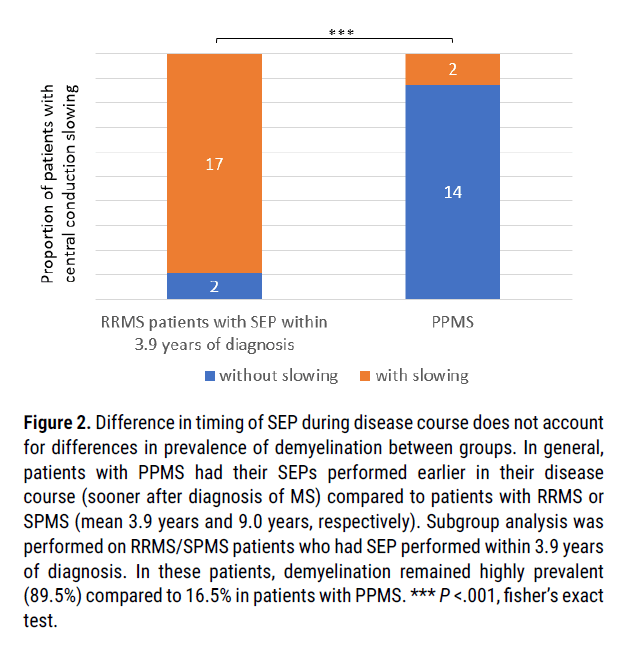
Figure 2: Difference in timing of SEP during disease course does not account for differences in prevalence of demyelination between groups. In general, patients with PPMS had their SEPs performed earlier in their disease course (sooner after diagnosis of MS) compared to patients with RRMS or SPMS (mean 3.9 years and 9.0 years, respectively). Subgroup analysis was performed on RRMS/SPMS patients who had SEP performed within 3.9 years of diagnosis. In these patients, demyelination remained highly prevalent (89.5%) compared to 16.5% in patients with PPMS. *** P <.001, fisher’s exact test.
Misclassification bias unlikely to account for between-group differences
An important consideration is that in this study, clinicians were not blinded to SEP results. As such, it is possible that the SEP findings may have influenced final subtype diagnoses, and therefore skewed the association of slowing and subtype classification through misclassification bias. Fortunately, a substantial number of patients had already received a subtype diagnosis prior to SEP, and consequently their SEP results could not influence their subtype diagnosis. Therefore, we performed subgroup analysis on these patients (diagnosis of RRMS at time of SEP, diagnosis of SPMS at time of SEP, and diagnosis of PPMS at time of SEP) and found that the between group differences remained unchanged. As such, it is unlikely that the differences in demyelination between groups is due to SEP results influencing final subtype diagnosis.
In this analysis of SEP data collected from patients with MS, CNS conduction slowing was highly prevalent in patients with RRMS or SPMS; however, measurable CNS conduction slowing was relatively rare in patients with PPMS. As CNS conduction slowing is a well-established marker of white matter demyelination, this suggests that the overall prevalence of clinically significant white matter demyelination is low in patients with PPMS. While it remains likely that some white matter demyelination is still present to a lesser extent (i.e. not identified by SEP) in patients with PPMS, our data suggest that white matter demyelination is unlikely to be the driver of axonal loss and disability accumulation in patients with primary progressive MS, although chronic white matter demyelination may still contribute to underlying neurodegeneration and disability accumulation in patients with SPMS. This has important clinical implications and suggests that therapeutic strategies aimed at ameliorating disease progression (including remyelination strategies) may have differential efficacy in patients with PPMS compared to patients with SPMS.
There are multiple data supporting a decrease in white matter demyelination in patients with PPMS. A previous histologic analysis by Tallantyre et al. [16] compared cervical cord sections from patients with SPMS and PPMS (with similar levels of motor disability), and found similar overall axonal loss in the corticospinal tract between groups, but significantly less grey and white matter demyelination in patients with PPMS compared to patients with SPMS. Additionally, radiologic studies have found similar overall whole brain atrophy despite lower T2-hyperintense and T1- hypointense lesion volumes in patients with PPMS compared to SPMS, again supporting the idea that axonal loss may be independent of demyelination in patients with PPMS [17]. Other pathologic comparisons also suggest decreased white matter demyelination in patients with PPMS compared to SPMS, although these found high levels of cortical demyelination in both PPMS and SPMS patients; which is likely not detectable by SEP [18,19]. Of interest, our findings differ from a study by Djuric et al. [20] which was established to compare the diagnostic power in MS (not subtypes) of Multimodal Evoked Potentials (MEP) versus MRI. This study was not set up to study SEPs in different subtypes of MS and reported prolonged SEP latencies in the majority of Serbian patients with PPMS. However, they reported only overall latency values (which included peripheral nerve conduction times) as opposed to central conduction values calculated here. In summary, it should be very clear that Djuric was not studying types of MS with SEP, but only comparing SEP, MEP and MRI.
There are limitations to this study. The potential for confounding bias and clinician bias skewing the data remains possible, although our subgroup analysis argues against timing of SEP in disease course influencing SEP results, and similarly argues against the idea that SEP results may have influenced MS subtype diagnosis. Another consideration is that while SEPs are a well-established marker of white matter demyelination throughout the afferent somatosensory pathway, they are unable to pick up demyelination in other tracts, including motor and visual pathways. Therefore, a normal SEP does not rule out the possibility of demyelination in other white matter tracts of the CNS. However, the histopathological analysis by Tallantyre et al. [16] found decreased demyelination in the motor cervical corticospinal tract in patients with PPMS compared to patients with SPMS, supporting the idea that decreased demyelination in patients with PPMS is not specific to afferent somatosensory pathways.
Another limitation is the length of data collection. Data collection for this study occurred over a period of 11 years (from 1999 to 2010). While a prolonged long data collection period may increase reporting bias, this study used the same SEP equipment, data collection protocols, and SEP central readers over the course of the study. Because all protocols remained consistent, we believe that the bias is limited and did not affect data analysis.
These data also suggest that SEP may be a useful strategy to categorize patients into RRMS or PPMS early in disease course, and hopefully lead to earlier use of subtype-specific disease modifying therapies. However, further work is required to investigate the predictive values of SEP when obtained very early in the disease. In addition, SEP are time-intensive endeavors requiring specialized equipment and training to implement and interpret, and patients and clinicians would benefit from a more efficient SEP approach prior to widespread use.
Therefore, SEP testing may be useful to help determine subtype of MS in those patients without a clear clinical diagnosis, which could aid in selection of DMT and clinical trials [21]. In particular, SEP testing could also help determine which patients with MS might benefit from future therapies that promote remyelination. Our initial studies, and others, suggest that tibial nerve stimulations, as opposed to median nerve stimulations, may have superior diagnostic utility, likely because the tibial electrical potential must travel through a longer portion of the spinal cord. Further work should be done to assess the predictive diagnostic capability of tibial nerve stimulations.
Many methods have been used to assess the various aspects of MS disease course. This includes the use of Diffusion Tensor MRI to assess the neurologic progression of PPMS, multimodal evoked potentials to determine status of MS, physical changes in MS, and as a biomarker [17,22-24]. While many methods have been used, current literature examining the use of SEP to assess MS diagnostic subtype is limited and other methods (such as DT MRI) are too complex for routine clinical use when compared to SEPs.
The study we present was able to significantly assess the diagnostic type (demyelinating or axonal) even early in the patient’s MS Course and is more cost-effective than other methods such as MRI.
Our results indicate that patients with PPMS have a significantly lower prevalence of CNS white matter demyelination compared to patients with RRMS or SPMS, and that this can be measured with SEP. This finding has important implications for underlying disease pathogenesis and suggests that potential therapeutic strategies targeting disability progression may have differential efficacy in patients with PPMS compared to SPMS. Finally, we believe that SEP testing can be used clinically with MS patients because it can provide early-disease subtype diagnosis, which has significant implications for medication and treatment selection.
The authors declare they have no competing interests.
This research was facilitated by the UW Medical Center policy to encourage innovative care of patients and funded by National Institute on Disability and Rehabilitation Research (NIDRR) Grant H133B080025 through the MS Rehabilitation Research and Training Center at University of Washington.
Aspects of this study have been presented in abstract form at the Consortium of Multiple Sclerosis Center Annual Meeting, May 30th, 2019; Seattle, WA, USA.
Citation: George H Kraft, Katie T Singsank, Sarah B Simmons and James D Bowen. A Clinical Perspective: Somatosensory Evoked Potentials May Suggest MS Type and Care Management. J Mult Scler (Foster City), 2021, 8(4), 421.
Received: 16-Mar-2021 Published: 27-Apr-2021, DOI: 10.35248/2376-0389.21.8.241
Copyright: © 2021 Singsank KT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This research was facilitated by the UW Medical Center policy to encourage innovative care of patients and funded by National Institute on Disability and Rehabilitation Research (NIDRR) Grant H133B080025 through the MS Rehabilitation Research and Training Center at University of Washington