Research Article - (2021) Volume 10, Issue 6
The Underrepresentation of Minorities and Non-Generalizability of Breast Cancer Clinical Trials?
Clyde CS1,
Yazzie GA1,
Cayatineto HW1,
Grunther B2 and
Joseph Angel de Soto1*
*Correspondence:
Joseph Angel de Soto, Laboratory of Pharmacogenetics and Health Care Disparities, School of STEM, New York,
United States,
Email:
Author info »
Abstract
Introduction: This year 43,000 women will die from breast cancer in the United States. African Americans and Native Americans though less
likely to get breast cancer, once diagnosed they are much more likely to
die from breast cancer. This increased death rate may in part be due to
the non-generalizability of breast cancer clinical trials. In this study, we
evaluate the participation of ethnic minorities from breast cancer clinical
trials.
Methodology: In this study, fifty-six breast cancer clinical trials
completed in the last ten years in the United States were evaluated for the
inclusion of ethnic minorities in the breast cancer clinical trials.
Results: Only 21% of breast cancer clinical trials include information
on ethnicity in the methodology while only 7% provided any information
on the effect or toxicity of the therapeutic intervention in minority groups
while 100% report the results for Whites. Though Whites only make up
60.1% of the population, they were 87.5% of the clinical trial participants
while African Americans were 6.2%, Hispanics 3.1%, Asians 2.9% and
Native Americans were 0.2% of the participants.
Conclusion: Racial minorities have been underrepresented in breast
cancer clinical trials which may contribute to unnecessarily high death
rates in these groups while suggesting limited generalizability of breast
cancer clinical trials.
Keywords
Breast cancer • Clinical trials • Native Americans • Health care
disparities • Racism • Pharmacogenetics • Non-generalizability • Exclusion
Introduction
One out of 8 women will get breast cancer in her lifetime. Each year
in the United States 282,000 women will be diagnosed with breast cancer
representing 14.8% of the new cancers. Forty-three thousand women will
die this year of breast cancer [1]. Whites have the highest incidence of
breast cancer at 131.8 per 100,000 females per year. This is in part due to
White women having a higher statistical rate of alcoholism, drug usage,
and a later age of first child than women of other ethnic groups [2-4].
All of which increase breast cancer rates. These facts become important
later. African Americans, who have a lower incidence of breast cancer
than Whites, have a higher death rate than Whites [5]. Once a person is
diagnosed with breast cancer their chance of dying if white is 15%, African
Americans 21.9%, Hispanics 13.7%, Asians 11.1%, and Native Americans
18.1%. Thus, African and Native Americans are at increased risk of death
when they get breast cancer though both groups having a lower incidence
of breast cancer. Breast cancer is treated depending on the type of breast cancer it is whether estrogen receptor positive or negative, progesterone
receptor positive or negative, and her2/neu positive or negative along
with the stage of the cancer I, II, III or IV [5]. These standard treatments
have been derived from clinical trials which are funded by the National
Institutes of Health (NIH) and/or the Pharmaceutical Industry with the
Food and Drug Administration approving or not approving the therapeutic
intervention.
Due to molecular differences in the cell cycle, cell differentiation,
genetic and epigenetics factors the type of breast cancer plays a heavy role
in determining the outlook for the disease. For instance, African American
women tend to have a higher rate of the most aggressive type of breast
cancer, triple negative that presents with the absence of the estrogen,
progesterone and her2/neu receptors [6]. The presentation of a higher risk
type of breast cancer has been used to explain away the higher risk of
death among African American women from breast cancer. Yet, Native
American women who also have a higher risk of death once they have
breast cancer do not present with the higher risk forms of breast cancer
yet have a higher death rate. It can even be argued that they present with a
more benign form of breast cancer than Whites. Thus, perhaps part of the
problem is how therapeutic interventions are determined and approved in
the United States. In this retrospective study we look at the inclusion and
exclusion of ethnic minorities in breast cancer clinical trials.
Methodology
In this study, breast cancer clinical trials performed within the United
States within the past ten years were selected by searching PubMed and
using the terms breast cancer, clinical trials, and study. Seventy-five papers
were then screened to ensure that 1) they were clinical trials; 2) they were
performed in the United States; 3) that the number or participants were
clearly defined; and 4) published within the past ten years. Fifty-seven peer
reviewed papers met the inclusion criteria [7-62]. These papers were then
evaluated for the inclusion of ethnic minorities in the methods and results
sections of the paper. These fifty-six clinical trials had an aggregate of
196,662 participants.
Results and Discussion
Fifty-seven clinical trials were evaluated for the inclusion or exclusion
of racial minorities in breast cancer clinical trials. Of the 56 clinical
trials only 21 reported the ethnic characteristics of the participants in
the methodology while, only 5 provided the results of the therapeutic
intervention on ethnic minorities. These 5 clinical trials who reported
the result of the therapeutic information were only two trials which had
sufficient numbers of ethnic minorities to provide useful information on
these populations of the intervention. Whites who make up 60.1 of the
population were 87.5% of the population. Hispanics who make up the
largest ethnic minority in the United States are 18.5% of the population
but only 3.1% of the breast cancer clinical trial participants. African
Americans who are 13.4% of the population made up only 6.2% of the
clinical trial participants. Asians and Native Americans who make up 5.9%
and 1.5% of the population had a representation of only 3.1% and 0.2% of
the breast cancer trial participants (Figures 1a and 1b). The participation
of ethnic minorities may actually be lower than reported as 63% of the
breast cancer clinical trials did not report the ethnic break-down of the
participants. The actual participation in clinical trials by Hispanics may
be as low as 1.6%, African Americans 3.2%, Asians 1.5%, and Native
Americans 0.14%.
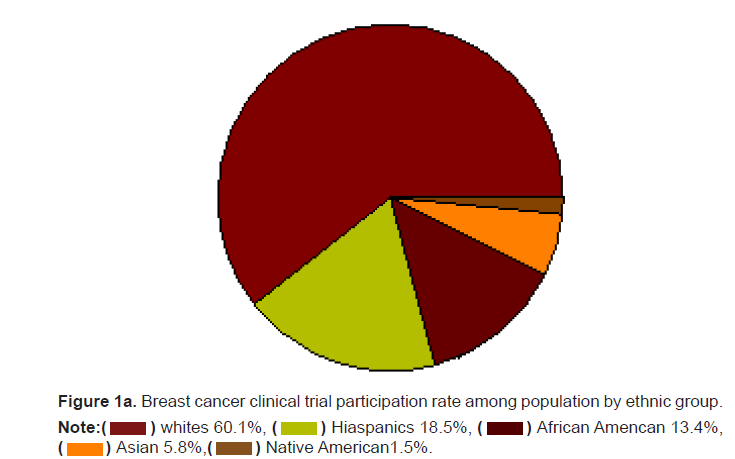
Figure 1a: Breast cancer clinical trial participation rate among population by ethnic group.
Note: African Amencan 13.4%,
African Amencan 13.4%, Native American1.5%.
Native American1.5%.
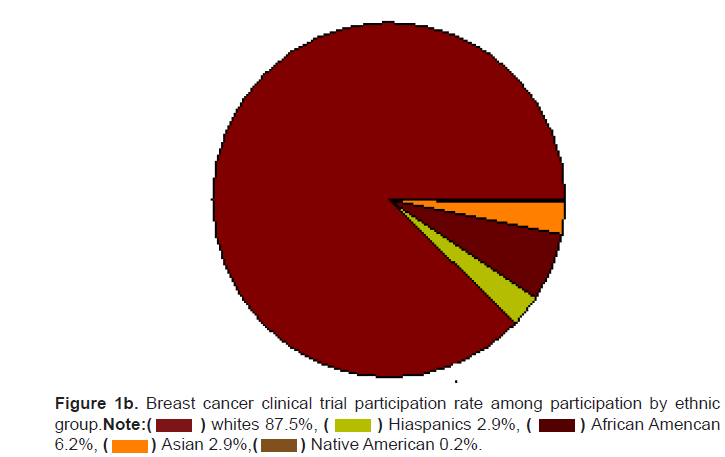
Figure 1b: Breast cancer clinical trial participation rate among participation by ethnic group.
Note: African Amencan 6.2%,
African Amencan 6.2%, 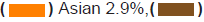
In order to have clinical trials that are generalizable to the population
as a whole they must include not only representatives from ethnic
minorities in significant numbers in the trial but, report on how the
therapeutic intervention acted on different ethnic groups. Here we see
that the overwhelming number of breast cancer clinical trials did not even
bother to report the ethnic background of the participants and even fewer
reported how the intervention affects ethnic minorities. Though ethnic
minorities make up 40% of the population they only represent 13% of the
breast cancer clinical trial participants with perhaps less than 2% providing
any useful information of the therapeutic intervention efficacy or toxicity
on minority populations. In a country where we speak of women’s rights
apparently in health care and research it only applies to White Women as
Hispanic, Native American, and African women are underrepresented and
seem not to count as they are deemed irrelevant in clinical trials and as
shown in this paper this applies to breast cancer as well.
In a country where the myth is that hard work and good conduct are
rewarded, we see that this is not true as White women tend to get breast
cancer at the highest rates due to lifestyle choices especially those in the
earlier years of life recalling that cancers develop over decades [63,64].
Thus, what one did twenty years ago or more determine today’s cancer
due to the eventual overcoming of the individuals DNA repair mechanisms
that preserve genomic integrity [65]. On the other hand, ethnic minorities
die at higher rates of breast cancer in part due to exclusion from medical
research based on skin color! To change this minority community must
step up and provide their own doctors, scientists, and researchers to
address this health care disparity and to change the future for the better.
This will also help the minority community regain trust in health care
providers and researchers and improve community engagement [66].
Conclusion
There is a total disregard for the life’s, well-being, and health of ethnic
minorities by the pharmaceutical industry, the federal government, and
medical researchers in the United States. Racial minorities have been
underrepresented in breast cancer clinical trials which may contribute
to unnecessarily high death rates in these groups suggesting limited
generalizability of breast cancer clinical trials. The lack of representation
of minorities in breast cancer clinical trials represents and easily solvable
problem resulting from systematic racism again non-whites in the United
States. Hispanics and Native Americans are clearly the most discriminated
against groups in clinical research followed by African Americans in breast
cancer clinical trials and research. In breast cancer trials, racial minorities
have been provided with insufficient or inadequate representation which
has led to more number of death rates which is unnecessary. While they
managed to limit the general ability for the same.
References
- National Cancer Institute. “Cancer stat facts: Female breast cancer.” (2021).
- Witbrodt, J., et al. “Racial/ethnic disparities in alcohol-related problems: Differences by gender and level of heavy drinking.” Alcohol Clin Exp Res 38.6(2014):1662-1670.
- Luczak, S.E., et al., “A review of the prevalence and co-occurrence of addictions in US ethnic/racial groups: Implications for genetic research.” Am J Addict 26.5(2017) 424-436.
- Martin, J.A., et al. “Births: Final data for 2018.” National Vital Stat Rep 68.13(2019).
- Dietze, E.C., et al. “Triple-negative breast cancer in African-American women: Disparities versus biology.” Nat Rev Cancer 15.4(2015):248-254.
- National Cancer Institute. “Cancer stat facts: Female breast cancer subtypes.” (2021).
- Bardia, A., et al. “Sacituzumab govitecan in metastatic triple-negative breast cancer.” N Engl J Med 384(2021):1529-1541.
- Tutt, A.N.J., et al. “Adjuvant olaparib for patients with BRCA1- or BRCA2- mutated breast cancer.” N Engl J Med 384(2021):2394-2405.
- Schmid, P., et al. “Pembrolizumab for early triple-negative breast cancer.” N Engl J Med 382(2020):810-821.
- Murthy, R.K., et al. “Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer.” N Engl J Med 382(2020):597-609.
- Modi, S., et al. “Trastuzumab deruxtecan in previously treated HER2-positive breast cancer.” N Engl J Med 382(2020):610-621.
- Baselga, J., et al. “Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(2012):520-529.
- Mehta, R.S., et al. “Overall survival with fulvestrant plus anastrozole in metastatic breast cancer.” N Engl J Med 380(2019):1226-1234.
- Verma, S., et al. “Trastuzumab emtansine for HER2-positive advanced breast cancer.” N Engl J Med -367(2012):1783-1791.
- Im, S.A., et al. “Overall survival with ribociclib plus endocrine therapy in breast cancer.” N Engl J Med 381(2019):307-316.
- Finn, R.S., et al. “Palbociclib and letrozole in advanced breast cancer.” N Engl J Med 375(2016):1925-1936.
- Turner, N.C., et al. “Albociclib in hormone-receptor-positive advanced breast cancer.” N Engl J Med 373(2015):209-219.
- Minckwitz, G.V., et al. “Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer.” N Engl J Med 377(2017):122-131.
- Tolaney, S.M., et al. “Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer.” N Engl J Med 372(2015):134-141.
- Slamon, D.J., et al. “Overall survival with ribociclib plus fulvestrant in advanced breast cancer.” N Engl J Med 382(2020):514-524.
- Hortobagyi, G.N., et al. “Ribociclib as first-line therapy for hr-positive, advanced breast cancer.” N Engl J Med 375(2016):1738-1748.
- Bao, T., et al. “Acupuncture for breast cancer-related lymphedema: A randomized controlled trial.” Breast Cancer Res Treat 170.1(2018):77-87.
- Zick, S.M., et al. “Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial.” Breast Cancer Res Treat 161.2(2017):299-310.
- Chlebowski, R.T., et al. “Dietary modification and breast cancer mortality: Long-term follow-up of the women's health initiative randomized trial.” J Clin Oncol 38.13(2020):1419-1428.
- Pu, M., et al. “Research-based PAM50 signature and long-term breast cancer survival.” Breast Cancer Res Treat 179.1(2020):197-206.
- Ligibel, J.A., et al. “Randomized trial of a physical activity intervention in women with metastatic breast cancer.” Cancer 122.8(2016):1169-1177.
- Byrne, C., et al. “Mammographic density change with estrogen and progestin therapy and breast cancer risk.” J Natl Cancer Inst 109.9(2017):djx001.
- Sonnenblick, A., et al. “Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: Analysis from the ALTTO phase iii randomized trial.” J Clin Oncol 35.13(2017):1421-1429.
- Hortobagyi, G.N. “Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: A review of subgroup analyses from the MONALEESA-2 trial.” Breast Cancer Res 20.1(2018):123.
- Lee, K., et al. “Feasibility of high intensity interval training in patients with breast cancer undergoing anthracycline chemotherapy: A randomized pilot trial.” BMC Cancer 19.1(2019):653.
- O'Shaughnessy, J., et al. “Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2-advanced breast cancer in the randomized MONALEESA-2 trial.” Breast Cancer Res Treat 168.1(2018):127-134.
- Ridner, S.H., et al. “A randomized trial evaluating bioimpedance spectroscopy versus tape measurement.” Ann Surg Oncol 26.10(2019):3250-3259.
- Swain, S.M., et al. “Incidence and management of diarrhea in patients with HER2-positive breast cancer treated with pertuzumab.” Ann Oncol 29.7(2018):1607.
- Guglin, M., et al. “Lisinopril or coreg CR in reducing cardiotoxicity in women with breast cancer receiving trastuzumab: A rationale and design of a randomized clinical trial.” Am Heart J 188(2017):87-92.
- Gucalp, A., et al. “Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer.” Clin Cancer Res 19.19(2013):5505-5512.
- Soliman, H., et al. “Mammaprint guides treatment decisions in breast cancer: Results of the impact trial.” BMC Cancer 20.1(2020):81.
- Regan, M.M., et al. “Concurrent and sequential initiation of ovarian function suppression with chemotherapy in premenopausal women with endocrine-responsive early breast cancer: An exploratory analysis of TEXT and SOFT.” Ann Oncol 28.9(2017):2225-2232.
- Regan, M.M., et al. “Adjuvant treatment of premenopausal women with endocrine-responsive early breast cancer: Design of the text and soft trials.” Breast 22.6(2013):1094-1100.
- Harvie, M., et al. “Breast cancer risk status influences uptake, retention and efficacy of a weight loss programme amongst breast cancer screening attendees: Two randomized controlled feasibility trials.” BMC Cancer 19.1(2019):1089.
- Lipton, A., et al. “Osteoporosis therapy and outcomes for postmenopausal patients with hormone receptor-positive breast cancer: NCIC CTG MA.27.” Cancer 123.13(2017):2444-2451.
- Chalasani, P., et al. “A phase I clinical trial of bavituximab and paclitaxel in patients with HER2 negative metastatic breast cancer.” Cancer Med 4.7(2015):1051-1059.
- Morris, P.G., et al. “Phase II study of paclitaxel and dasatinib in metastatic breast cancer.” Clin Breast Cancer 18.5(2018):387-394.
- Smyth, E.N., et al. “Patient-reported pain and other quality of life domains as prognostic factors for survival in a phase III clinical trial of patients with advanced breast cancer.” Health Qual Life Outcomes 25(2016):14:52.
- Xu, J., et al. “A phase ii trial of cabozantinib in hormone receptor-positive breast cancer with bone metastases.” Oncologist 25.8(2020):652-660.
- Park, N.J., et al. “Cardiovascular disease and mortality after breast cancer in postmenopausal women: Results from the women's health initiative.” PLoS One 12.9(2017):e0184174.
- Jimenez, R.B., et al. “Phase II study of proton beam radiation therapy for patients with breast cancer requiring regional nodal irradiation.” J Clin Oncol 37.30(2019):2778-2785.
- Vinayak, S., et al. “A clinical trial of lovastatin for modification of biomarkers associated with breast cancer risk.” Breast Cancer Res Treat 142.2(2013):389-398.
- Wu, T., et al. “Hemoglobin A1c levels modify associations between dietary acid load and breast cancer recurrence.” Nutrients 12.2(2020):578.
- Villaseñor, A., et al. “Postdiagnosis C-reactive protein and breast cancer survivorship: Findings from the WHEL study.” Cancer Epidemiol Biomarkers Prev 23.1(2014):189-199.
- Zhao, S., et al. “Sex hormone associations with breast cancer risk and the mediation of randomized trial postmenopausal hormone therapy effects.” Breast Cancer Res 16.2(2014):R30.
- Going, C.C., et al. “Vitamin D supplementation decreases serum 27-hydroxycholesterol in a pilot breast cancer trial.” Breast Cancer Res Treat 167.3(2018):797-802.
- Cowherd, S., et al. “A phase II clinical trial of weekly paclitaxel and carboplatin in combination with panitumumab in metastatic triple negative breast cancer.” Cancer Biol Ther 16.5(2015):678-683.
- Stagl, J.M., et al. “A randomized controlled trial of cognitive-behavioral stress management in breast cancer: Survival and recurrence at 11-year follow-up.” Breast Cancer Res Treat 154.2(2015):319-328.
- Bardia, A., et al. “Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer.” Breast Cancer Res Treat 131.3(2012):907-914.
- Bramwell, V.H.C., et al. “Assessment of osteopontin in early breast cancer: Correlative study in a randomised clinical trial.” Breast Cancer Res 16.1(2014):R8.
- Karlsson, P., et al. “Timing of radiation therapy and chemotherapy after breast-conserving surgery for node-positive breast cancer: Long-term results from international breast cancer study group trials VI and VII.” Int J Radiat Oncol Biol Phys 96.2(2016):273-279.
- Kesler, S.R., et al. “Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy.” Brain Imaging Behav 7.4(2013):501-510.
- Lynce, F., et al. “SAFE-HEaRt: Rationale and design of a pilot study investigating cardiac safety of her2 targeted therapy in patients with her2-positive breast cancer and reduced left ventricular function.” Oncologist 22.5(2017):518-525.
- Brown, J.C., & Schmitz, K.H. “Weight lifting and appendicular skeletal muscle mass among breast cancer survivors: A randomized controlled trial.” Breast Cancer Res Treat 151.2(2015):385-92.
- Gingras, I., et al. “Regional nodal irradiation after breast conserving surgery for early her2-positive breast cancer: Results of a subanalysis from the altto trial.” J Natl Cancer Inst 109.8(2017):djw331.
- Howell, A., et al. “Razor: A Phase ii open randomized trial of screening plus goserelin and raloxifene versus screening alone in premenopausal women at increased risk of breast cancer.” Cancer Epidemiol Biomarkers Prev 27.1(2018):58-66.
- Schwartzberg, L.S., et al. “A phase I/Ib study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer.” Clin Cancer Res 23.15(2017):4046-4054.
- Liu, Y., et al. “Links between alcohol consumption and breast cancer: A look at the evidence.” Womens Health (Lond) 11.1(2015):65-77.
- Greene, K.M., & Maggs, J.L. “Drinking, social abstaining, and refusing invitations: Demographic differences persist across college.” Alcohol Clin Exp Res 44.1(2020):203-211.
- Basu, A.K. “DNA damage, mutagenesis and cancer.” Int J Mol Sci 19.4(2018):970.
- Holzer, J.K., et al. “Why we need community engagement in medical research.” J Investig Med 62.6(2014):851-855.
Author Info
Clyde CS1,
Yazzie GA1,
Cayatineto HW1,
Grunther B2 and
Joseph Angel de Soto1*
1Laboratory of Pharmacogenetics and Health Care Disparities, School of STEM, New York, United States
2Department of Information Technology, Dine College, Tsaile, Arizona, United States
Citation: Clyde CS, et al. The Underrepresentation of Minorities and Non-Generalizability of Breast Cancer Clinical Trials? J Biol
Today's World, 2021, 10(6), 001-004.
Received: 20-Aug-2021
Published:
10-Sep-2021
Copyright: © 2021 Clyde CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use,
distribution, and reproduction in any medium, provided the original author and source
are credited.

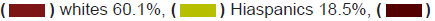 African Amencan 13.4%,
African Amencan 13.4%,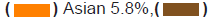 Native American1.5%.
Native American1.5%.
 African Amencan 6.2%,
African Amencan 6.2%,