Review - (2021) Volume 13, Issue 5
Pen needles are essential for drugs injections using pen devices. The use of injection pens with needles sometimes causes accidental puncture. Accidental needle stick injuries (NSI) afflict not only patients, but also impact healthcare professionals who administer medications. The introduction of safety pen needles, with incorporated sharps injury prevention feature, is an advantage over the traditional pen needles reducing the risk of injury and the spread of infectious diseases. This study presents the results of the simulated clinical use study evaluating the safety of the use of DropSafe safety pen needle (SPN), in the prevention of NSI and evaluating the user’s satisfaction with regards to the handling characteristics of the product.
Accidenstal blood exposure; Diabetes; Healthcare ;Medical device; Needle stick injury; pen needle ;Safety pen needle; Sharps injury prevention; Blood borne pathogen; Protection
ABE: Accidental Blood Exposure; HCP: Healthcare Professionals; NSI: Needle Stick Injuries; NHCP: Non-Healthcare Professionals; IFU: Instructions For Use; SPN: Safety Pen Needle
Accidental Blood Exposure (ABE) is defined as an accident associated with exposure to blood, bloody fluids or other fluids. Examples of the modes of exposure include percutaneous injuries, mucous membrane exposure, and non-intact skin exposure delivered by aerosolization, splash-type events, and piercing of the integument with contaminated objects [1]. ABEs are an inherent risk in that the handling of needles or sharp objects that may be contaminated with blood or other body fluids, and expose healthcare professionals (HCP) and non-healthcare professionals (NHCP) to the risk of serious infection [2,3]. Injuries from non-intentional piercing of the skin with a needle [needle stick injury (NSI)] can occur at various times including during the use of a device with a needle: at the stage of introduction of the needle, at the withdrawal of the needle from the tissue, or other contaminated device, and during the disposal of a device with a needle.
A medical device with a sharps injury prevention feature is a device designed with a component or attachment, either active or passive, that protects the user from a sharps injury. The Drop Safe safety pen needle (SPN) (manufactured by HTL-Strefa, Poland) is designed in a way that sharps injury prevention feature is incorporated as integrated component of finished device.
Pen needles are essential for insulin injections using pen devices. The use of insulin injection pens with needles sometimes causes accidental puncture. Accidental needle stick injuries (NSI) afflict not only diabetics, but also impact health care professionals who inject insulin. NSI may occur while uncapping an insulin needle, injected into a person, especially if a skin fold is used, or while recapping. NSI can occur from exposure to the front tip of the needle, or to the back end (cartridge end) of a pen needle. The introduction of SPNs is an advantage over the traditional pen needles and may reduce the risk of injury and the spread of infectious diseases.
Although pen needles with an automatic recapping ability is widely used in clinical practice, there is still no much literature and comprehensive evaluations of this kind of medical devices.
The manuscript of the article presents the results of the simulated clinical use study evaluating the safety of the use of Drop Safe SPN, developed by HTL-Strefa S.A., in the prevention of NSI and evaluating the user’s satisfaction with regards to the handling characteristics of the product.
Drop Safe SPN are sterile, single-use safety needles intended for use with pen injector devices for the injection of drugs. The device was designed to minimize the risk from accidental needle sticks with a used needle by application of a sharps injury prevention feature. Following use, the needle is locked out preventing reuse. The shield also serves to hide the needle before and after injection (Figure 1).The Drop Safe SPN is used by attaching it to the pen injector device to administer a drug subcutaneously. While inserting the needle into the skin at a 90° angle, the slider glides into the shield. While the slider glides into the shield, the safety feature is activated. Following injection, the slider glides back into its initial position, completely covering the needle where it remains locked. The red safety lock indicator tells the user that the safety lock has been activated. Once the SPN is in the locked mode, it cannot be reused. The SPN can be then detached from the pen injector device and disposed safely.
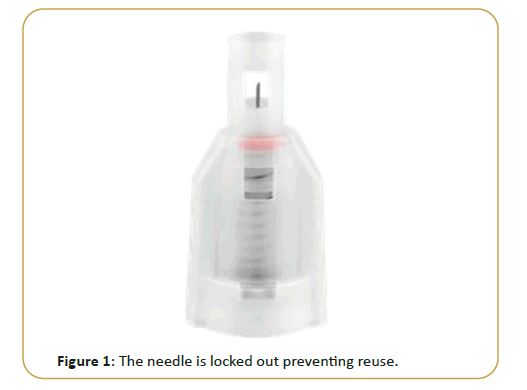
Figure 1: The needle is locked out preventing reuse.
A simulated clinical user study was designed to evaluate the safety of use of Drop Safe SPN in the prevention of needle stick injuries. The primary objective was to evaluate the number of injuries or non-activation of the safety feature reported by the evaluators for the device. Success was defined as a complete activation of the safety feature following injection without a NSI, whereas failure was defined as NSI or a non-complete activation of the safety feature following injection reported by the evaluators for each device.
The study was designed in accordance with US FDA Guidance for Industry and FDA Staff, “Medical Devices with Sharps Injury Prevention Features, Section 10, Simulated Clinical Use Testing” [4].
In accordance with the FDA recommendations, 540 safety devices (n=540) were tested. Acceptance criteria were defined in accordance with the FDA Guidance [5].
The study was performed in the US by a total of 30 evaluators [6]. The evaluators were composed of 20 non-healthcare professionals (NHCP) and 10 healthcare professionals (HCP) [7]. Of the 540 injections performed with Drop Safe SPN, 180 were performed by a HCP and 360 by a NHCP [8].
To mimic real clinical conditions of device use, HCPs performed injections with gloves and NHCPs without gloves [9]. Injections were performed using an orange. The use of fruit as a model mimicked a subcutaneous (SC) route of administration [10]. For each device, the evaluators were to administer the injection with the Drop Safe SPN and pen injector with a sterile, water-filled cartridge, strictly following the instructions for use [11,12 ].
The analysis of the injury onset and success of manipulations showed the following results:
• zero injuries and zero failures of the Drop Safe SPN performed by a HCP,
• zero injuries and zero failures of the Drop Safe SPN performed by a NHCP.
Of the 540 simulated injections performed with Drop Safe SPN, zero failures were observed and all manipulations were performed without a needle stick or without contact with the needle after injection [13,14]. In addition, the safety feature of the Drop Safe safety pen needle was activated successfully [15,16]. The failure rate in the validation was 0.6%. According to the tables given in the FDA guidance [17,18], these observations have determined that the primary objective of the simulated clinical user study was achieved for Drop Safe SPN (accepstance cristeria is less sthan 1%) [19,20].
This study not only confirmed the performance of the two versions of the sharps injury protection device but also gave the opportunity to evaluate instructions for use (IFU) for clarity and utility and to capture feedback from the evaluators on several aspects of the safety device and on. The evaluators completed the evaluation questionnaire, using 5-point Likert scale, in which 1=strongly disagree and 5=strongly agree. It was demonstrated (based on the combined rating for the two highest categories: strongly agree and agree) that:
- The label on the seal was legible and properly identified the length of the needle (100% of HCPs and 90% of NHCPs)
- The safety pen needles were easy to attach to the pen (70% of HCPs and 95% of NHCPs)
- Removing the outer cap was easy (100% of HCPs and NHCPs)
- The needle was visible through the viewing window (100% of HCPs and NHCPs)
- Priming test could be assessed through viewing window (100% of HCPs and 95% of NHCPs)
- Injection was easy (100% of HCPs and NHCPs)
- It was clear when safety feature was activated (100% of HCPs and 90% of NHCPs)
- Injection and safety feature activation could be easily performed using one hand (60% of HCPs and 65% of NHCPs)
- Removing needle from pen was easy (90% of HCPs and 100% of NHCPs)
- It was easy to put the needle into the sharps container (100% of HCPs and NHCPs)
- The written instructions were easy to understand (90% of HCPs and 100% of NHCPs
Taking into account the above-mentioned observations it was demonstrated that:
- No extensive training was needed to use Drop Safe safety pen needle effectively
- Injection and activation of the needle safety was easy
- Use of the safety feature was obvious
- It was clear when the safety feature was activated
- The safety feature activated only when injection was complete.
NSI is one of the most common health hazards in the healthcare setting. The World Health Organisation estimates about 3 million of the 35 million health care workers worldwide are exposed to blood-borne pathogens each year.
It is estimated that there are one million needle-stick injuries a year. Other estimates put the number of NSI per worker healthcare between 0.1 and 0.64 or even be as high as 0.98 per year. Based on the above figures, it is argued that the number of NSIs alone would be between 800,000 and 5,120,000 per year, however, 600,000 reported injuries and 600,000 unreported injuries are more likely. Accidents of this kind were most common in nurses working in dialysis units, emergency rooms, GP surgeries, surgical wards, and operating rooms.
Independent studies showed that the majority of these potentially fatal injuries can be avoided using a combination of training, safer working practices and medical technology incorporating safety features, e.g. needles with automatic protective sheaths .
The most effective way to reduce the NSI prevalence among healthcare workers is to use safety equipment which is easy to use with safety mechanisms that are easy to activate. Pen needles are often implicated in the risk of sharps injury, as they are used widely by diabetic patients, as well as by healthcare workers giving subcutaneous injections to patients. Pen needles with automatic protective safety feature have been found to minimize the risk of exposure to blood-borne infections, such as hepatitis B virus, hepatitis C virus and human immunodeficiency virus, associated with NSIs, without any related adverse event. Recent findings revealed that patients with type 1 diabetes (T1DM) can experience improvement in the level of pen needles usability, HbA1c values, and hypoglycemia after usage of the SPN. The SPN significantly improved usability and reduced the fear of insulin selfinjections amongst T1DM patients compared to conventional pen therapy.
The performance and its ease of use, ergonomics and functionality of the device were confirmed and appreciated by users during the simulated clinical user study. Drop Safe SPN was considered to be an effective needle stick protection device, without use problems, which allows an injection to be performed using a one-hand technique, and which does not need extensive training for correct manipulation.
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.