Research Article - (2021) Volume 10, Issue 4
Bioavailable Testosterone (BT) is henceforth well-known to be much more useful than Total Testosterone (TT) for interpretation. BT is mostly explored in case of suspicion of hypogonadism in men or hyperandrogenism in women. The reference technique to assay BT is ultracentrifugation, but Radio-Immuno Assay (RIA), which is much less expensive and easier to accomplish is the most widely used technique in practice. Several calculations are available but the one that give the better results seem to be the optimized calculation of the Vermeulen equation by Giton. Very few comparisons between BT assayed and BT calculated have been made in routine, hence our study. This is a prospective study which has included 269 patients (whose 189 men) to compare both results in the frame of matched data. Different subgroups, depending on gender, age, TT values, SHBG values and albumin values have been studied. Although BT calculated values seem to be significantly higher to those in RIA, correlation appear to be excellent in men with a correlation coefficient established at 0.92, but not in women. BT calculated appear therefore to be an excellent alternative to assay, and has obvious other advantages: It is free, allow faster result delivery and a blood test requiring one tube less for patients.
Biochemistry • Hormonology • Bioavailable • Testosterone • Radio- Immuno Assay
Bioavailable Testosterone (BT) corresponds to the mobilizable fraction of testosterone, which in practice corresponds to Free Testosterone (FT), which of course can diffuse freely, but also testosterone bound to albumin because this can be dissociated very easily [1]. BT therefore depends mainly on the level of SHBG (Sex Hormone Binding Globulin) because if it increases-as it is strongly the case in the context of aging-, BT will decrease accordingly (at equal Total Testosterone (TT)). BT, much more than TT, undergoes a very significant decline with aging [2,3]. Usually, the estimate of the level of BT can be done either by dosage or by calculation [4]. There are two main indications for exploring the androgenic route: suspicion of hypognadism in men and suspicion of hyperandrogenism in women.
Currently, the diagnosis of hypogonadism in men requires the presence of symptoms and signs suggestive of testosterone deficiency. The symptoms most associated with hypogonadism are low libido and erectile dysfunction [5]. To make a diagnosis of hypogonadism, one or more of these symptoms must be corroborated by low testosterone, low libido by itself being insufficient to make a diagnosis of hypogonadism. Biochemical hypogonadism is established if BT is less than 70 ng/dL (2.43 nmol/L) or if the TT is less than 300 ng/dL (10.40 nmol/L) [5]. Classically, primary hypogonadism (of testicular origin) is differentiated from secondary hypogonadism (of hypothalamic-pituitary origin). Primary or secondary hypogonadisms can occur at any age [5]. The causes of primary hypogonadisms (testicular causes-with high LH and FSH levels) are mainly represented by castration, testicular trauma, Klinefelter syndrome, orchitis, chemotherapy, or testicular radiotherapy. The causes of secondary hypogonadisms (hypothalamic-pituitary causes-with low LH and FSH levels) are mainly due to insufficient secretion of hypothalamic GnRH (Kallmann syndrome), pituitary/hypothalamic tumors, hypopituitarism or even pituitary surgery [6]. Hyperandrogenism in women corresponds to an abnormally high blood concentration of androgens (mainly testosterone). This is suspected in the event of clinically suggestive symptoms, with hirsutism at the top of the list. Other symptoms are possible like excess acne, seborrhea, alopecia, dysmenorrhea/amenorrhea and high cholesterol [7]. The consequences of hyperandrogenism are manifold and can be potentially serious or disabling; these include obesity, hypertension or infertility. Since hyperandrogenism is responsible for a high tolerance to insulin, type 2 diabetes can also develop. At the same time, the psychological consequences (anxiety and depression) are far from negligible, especially among adolescent girls and young women [8]. In 70%-80% of cases, hyperandrogenism is due to Polycystic Ovary Syndrome (PCOS). PCOS is suspected in the association of hirsutism and oligo-ovulation or even anovulation. Biologically there is a moderate increase in circulating androgen levels. The other causes are dominated by adrenal hyperplasia (congenital-due to 21 hydroxylase deficiency-or not), testosterone secreting tumors and certain drugs (anabolic steroids, danazol, metoclopramide, phenothiazines, progestins, methyldopa, and reserpine). Finally, it should be noted that there are idiopathic hirsutisms (20% to 30% of all hirsutisms) [9]. Hyperandrogenism is confirmed by TT and BT blood tests.
The historical estimate of the level of circulating androgens consisted of a single dosage: That of TT, due to the ease of performing and the lack of knowledge relating to the time. It is now clear that BT is of much greater interpretative interest than TT [10]. Indeed, the only fraction of testosterone which is easily mobilized and therefore usable by tissues corresponds to BT. It is not uncommon to observe dissociation between BT and TT, whether it is normal TT while BT is reduced, or TT decreased with BT that remains within the norm [11]. Suspicion of androgen deficiency, whether age-related or not, is the main indication for estimating BT [12]. According to the French Association of Urology, androgen deficiency is biologically proven when BT result is below 80 ng/dL (2.77 nmol/L) [13]. In suspected hyperandrogenism in women, BT estimate is less interesting due to the low circulating levels of TT physiologically, so even a minor elevation of TT may be readily detectable. Of course, in women, as in men, the dosage of BT is a better reflection of the reality on the ground, but it is not essential. The other indications for estimating BT are few, they are two in number: Adjustment of the dosage of a testosterone-based treatment and the suspicion of puberty disorders (precocious puberty or delayed puberty). In addition, BT, quite logically being the active fraction of testosterone, is inversely correlated with certain pathologies in which testosterone is protective such as hypertension, which is not the case with TT [14]. The purpose of determining whether or not the patient has androgen deficiency, and the initiation of Testosterone-Based Treatment (TRT), so as to compensate for this deficiency and thus prevent all the harmful consequences associated with it. However, although all the studies carried out recommend BT rather than TT to interpret a possible deficit, the guidelines for TRT of most learned societies are still based on the rates of TT. For instance, this is the case of ISSM (International Society for Sexual Medicine) in 2015 with an indication for TRT if TT is below 350 ng/dL (12.14 nmol/L), or for the American Association of Urology in 2018 (indication for TRT if TT<300 ng/dL or 10.40 nmol/L) [15]. However, these guidelines state that in theory BT is the most precise parameter, but that it is not widely used due to the lack of a widely accepted reference method to determine it [15].
Currently, three techniques for assaying BT are available: Equilibrium dialysis, ultracentrifugation and Radio-Immuno Assay (RIA) [16]. RIA is the historical assay technique, still widely used today, because it is much easier to perform and less expensive in terms of technical time and economically speaking. However, this gives less reliable results than the other two methods, with significantly lower results [16]. Steady- state dialysis is estimated by almost all authors as the gold standard for assaying BT. However, this is not widely used in practice because of its economic and above all technical costs which are very high [16,17]. Ultracentrifugation is an alternative to equilibrium dialysis because it gives substantially equivalent results, clearly more correct than those obtained in RIA [18-20]. Ultracentrifugation has the advantage of having a shorter technical time and a lower implementation cost than for equilibrium dialysis, and is therefore much more commonly used than the latter [16]. RIA is historically the first immunoassay technique developed (in the 1950s) to analyze very low concentrations of hormones in body fluids, and still has its place today, especially because of its low cost, its ease of use, high sensitivity and high specificity [21]. The principle is to create a competition between an antigen present in the sample (called “cold antigen” because not radiolabeled), the concentration of which we want to determine, and an antigen added exogenously (called “hot antigen” because radiolabeled), in a known quantity, with respect to a concentration of specific target antibodies known which will be added subsequently [21]. The most widely used radioactive label is iodine 125(125I); however other isotopes such as carbon 14 (C14) and tritium (H3) can also be used [22]. An antigen-antibody complex is then formed, this being due to the interaction between the epitopes of the antigen and the paratopes of the antibody. It involves four types of non-covalent bonds (hydrogen, electrostatic, hydrophobic, and van der waals forces) [22]. In practice, the antigen to be assayed is placed at the same time as the radiolabeled antigen on a plate coated with the specific antibody. Thus, the colder antigens there are in the sample, the less hot antigens can bind to the antibodies in presence. The competition for antibodies releases a certain number of labeled antigens, which could not be fixed. This quantity is proportional to the radiolabeled antigens/non-radiolabeled antigens ratio. A calibration curve can then be used (from a known standard) to determine the amount of cold antigens present in the patient's serum (or other body fluid) [21].
BT can also be calculated. There are several equations published by different authors in order to calculate BT as accurately as possible. We first have historically FAI (Free Androgen Index) published in 1985 and the Södergard equation [23,24]. FAI is sometimes used because of its ease of realization in order to give an approximative result, but on the other hand it is well known not to be very reliable and is therefore not recommended in routine, and especially for men [25]. The formula for this calculation is simply: FAI=Total Testosterone (nmol/L)/SHBG (nmol/L). The Södergard equation, originally published in 1982, is based on the results of TT, SHBG and albumin. It has undergone several evolutions in order to improve its performance. This was first of all the case with Vermeulen in 1999 who had the idea of adding an association constant for albumin [16]. Subsequently, Giton et al. in further improved this equation by introducing and optimizing an association constant for SHBG and the association constant for albumin [4]. This equation, which is commonly called the optimized equation of the Vermeulen equation, is therefore the most successful method of calculating BT and the closest to the results obtained by assay. The calculation formula is: BT=-b+√-b2+4aT/2Ks
where a=(1+Ka × Albumine) Ks and b=1+KaA+Ks(SHBG-TT).
In order to obtain BT concentrations as close as possible to assay values, the association constants of testosterone for SHBG (Ks) and for albumin (Ka) were optimized. The optimized values of Ka and Ks highlighted are: Ka=2.5 × 104 L/mol and Ks=1.9 × 109 L/mol. All the concentrations in this equation have to be in the same unit (mol/L usually). Finally, note that the French Association of Urology recommends estimating BT by calculation rather than by assay due to the vagaries of techniques for assaying bioavailable testosterone fraction [10].
This study is a prospective study based on a single cohort of patients. It took place from May 2018 to November 2020. These patients come from a single center: Amiens University Hospital. They mainly come from, but are not limited to, day care and full-time endocrinology departments. Our study concerns 269 patients including 189 men (70.26%) and 80 women (29.74%). The patients concerned are between 18 and 87 years old, with an average age established at 47.7 years The first quartile is established at 33 years old, the median at 48 and the third quartile at 63.
Sub-groups
We determined different subgroups of patients to evaluate the performance of calculation compared to RIA results for these subgroups in order to see if for certain subgroups the performance of the calculation is better than for others. These subgroups have been established depending on gender: men and women. And for men, we have determined other subgroups: according to age (under 50 and over 50), according to TT values (less than 188 ng/dL (6.52 nmol/L); from 188 to 684 ng/dL (normal values) and more than 684 ng/dL (23.72 nmol/L)), according to SHBG values (less than 10 nmol/L; 10 to 57 nmol/L (normal values) and more than 57 nmol/L), and depending on the albumin values (less than 32 g/L; from 32 to 48 g/L (normal values) and more than 48 g/L).
Protocol
Each patient, as part of routine, was collected by a nurse from the department to which he is affiliated. Three tubes were taken for each patient: Two dry tubes (preferably with separator gel) and a lithium heparinate tube. The first dry tube was sent to the Cerba laboratory (Saint- Ouen-l'Aumône, France) for determination of BT in RIA. On the second dry tube, TT and SHBG were assayed on Siemens Atellica IM module, while the lithium heparinate tube was assayed for albumin on the Siemens Atellica CH module, both in our laboratory, in the Amiens University Hospital.
Concerning TT, blood sample was collected in a dry tube (preferably with a separating gel). The sample was then sent to the laboratory at Ambient Temperature (TA). The stability of whole blood for the TT parameter is 72 hours at TA or at +5°C ± 3°C [24]. The primary tube is centrifuged according to our laboratory protocol (2250 g ± 200 g, 13 ± 2 min and +20°C ± 4°C). TT assay is performed on serum on the Atellica IM Siemens module. The serum is stable 72 hours at TA or at +5°C ± 3°C [24]. The test upstream uses a releasing agent (helper reagent) to release testosterone bound to binding proteins. The TT test is a competitive immunoassay. The TT in the sample competes with the acridinium ester labeled happen to bind to the total anti-testosterone monoclonal antibody, which is itself covalently bound to paramagnetic particles. After washing steps, the acid/base couple is added to oxidize the acridinium ester and thus cause the chemiluminescence reaction. The light emission generated is inversely proportional to the amount of TT present in the sample. The Siemens reference values for the TT parameter are ranged from 197 to 670 ng/dL (i.e. 6.85 to 23.23 nmol/L) for men under 50 years old, and from 188 to 684 ng/dL (i.e. 6.51 to 23.74 nmol/L) for men over 50 years old.
For SHBG, the blood sample was collected on a dry tube with a separating gel. The sample was then sent to the laboratory at TA. The SHBG parameter is stable for 72 hours at TA or at +5°C ± 3°C [25]. The primary tube was centrifuged according to the laboratory protocol (2250 g ± 200 g, 13 ± 2 min and +20°C ± 4°C). SHBG assay is performed on serum on the Siemens Immulite 2000 XPi machine. The serum is stable for 48 hours at TA or 7 days at +5°C ± 3°C. The SHBG test is a sandwich immunoassay. This test uses an anti-SHBG monoclonal antibody covalently linked to beads and an anti-SHBG polyclonal antibody labeled with alkaline phosphatase. The SHBG in the sample will bind to both the anti-SHBG antibody itself attached to the beads and the anti- SHBG antibody labeled with alkaline phosphatase. After washing steps, dioxetane is added and will be dephosphorylated by alkaline phosphatase to an unstable anion which will cause the chemiluminescence reaction. The light emission generated will be proportional to the amount of SHBG present in the sample. Normal values of SHBG for adult males range from 10 to 57 nmol/L.
Concerning albumin, the blood sample was collected on a lithium heparin tube. The sample was then sent to the laboratory at TA. The albumin parameter is stable in plasma for 24 hours at TA or at +5°C ± 3°C. The primary tube is centrifuged according to the laboratory protocol (2250 g ± 200 g, 13 ± 2 min and +20°C ± 4°C). Albumin is the protein with the highest concentration in blood plasma. Its synthesis is essentially hepatic and its half-life is 21 days. It has a major role in maintaining the oncotic pressure of plasma and as a transport protein. In our laboratory, albumin assay is performed in plasma on the Atellica CH Siemens automated system by colorimetry. The plasma is stable for 48 hours at TA or 7 days at +5°C ± 3°C. The method used is that of Doumas, Watson and Biggs by measuring fixation with the green dye of bromocresol. Plasma albumin binds to bromcresol green to form an albumin-bromcresol green complex, measured at the 596/694 nm equivalence point. The usual laboratory values, for an adult population free of pathology, are between 32 and 48 g/L.
To obtain BT by calculation, we used the optimized calculation of the Vermeulen equation by Giton et al. because this method of calculating the TB is the most successful and the closest to the results obtained by the reference assay [4]. Following the determination of albumin, SHBG and TT at the CHU, we were able to perform this calculation for each patient.
To obtain BT by RIA, after receiving and centrifuging the dedicated dry tube, the sample was put at refrigerated temperature (5°C ± 3°C) and then carried at this temperature by an approved transporter up to Cerba laboratory in Paris suburb. There, the sample underwent RIA to obtain BT.
Comparison techniques
BT values, whether obtained by calculation or by assay, do not follow a normal law according to the Shapiro-Wilk test (alpha=5%, p<0.0001). Accordingly, we resorted to non-parametric comparison tests. Thanks to our protocol, for each patient, we have a couple of results BT obtained by RIA/BT obtained by calculation. To analyze this matched date, we first focused on the total cohort study, with correlation test, Wilcoxon test and Kolmogorov-Smirnov test. Then, we focused on men and women with correlation and Wilcoxon test. Finally, among men, we analyzed correlation for every other subgroup we had created: According to age (aged less than 50 years old/aged more than 50 years old), TT values (<188 ng/dL; 188- 684 ng/dL and >684 ng/dL), SHBG values (<10 nmol/L; 10-57 nmol/L and >57 nmol/L) and albumin values (<32 g/L; 32-48 g/L and >48 g/L). For TT, SHBG and albumin values, these cutoffs have been decided corresponding to low, normal and high values respectively in our laboratory of the Amiens University Hospital.
We obtain albumin values between 19.2 and 54.2 g/L. The first quartile is at 40, the median at 43 and the third quartile at 45.1 g/L. The mean is 42.57 g/L (42.03-43.11(CI=95%)). We obtain TT values between 8 and 1666 ng/dL (0.28 to 57.76 nmol/L). The mean is established at 268.5 ng/ dL or 9.31 nmol/L (235.1-301.8(CI=95%)), the median at 200 ng/dL (6.93 nmol/L) with a first quartile evaluated at 38 ng/dL (1.32 nmol/L) and a third quartile at 418 ng/dL (14.49 nmol/L). Concerning SHBG, the mean of the population studied is established at 38.6 nmol/L (35.4-41.7(CI=95%)), for values between 7.6 and 180 nmol/L. The median is 31.9 nmol/L, the Q1 at 21.8 nmol/L and the Q3 at 45.6 nmol/L. BT values measured in RIA are between 9 and 586 ng/dL (0.31-20.32 nmol/L) with an average established at 73.3 ng/dL or 2.54 nmol/L (63.0-83.5(CI=95%)), a median at 46 ng/dL (1.59 nmol/L), Q1 at 12 ng/dL (0.42 nmol/L) and a Q3 at 98 ng/dL (3.40 nmol/L). BT values estimated by calculation are between 0.4 and 617.4 ng/dL (0.01-21.41 nmol/L) with an average established at 84.2 ng/dL or 2.92 nmol/L (71.8-96.6(CI=95%)), a median at 56.2 ng/dL (1.95 nmol/L), Q1 at 8.2 ng/dL (0.28 nmol/L) and a Q3 at 117.2 ng/dL (4.06 nmol/L) (Figure 1).
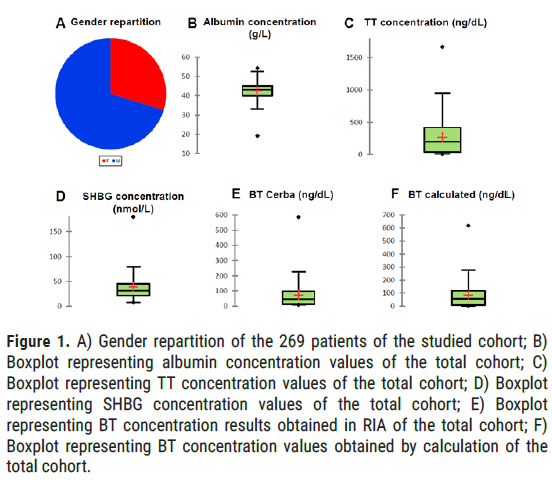
Figure 1: A) Gender repartition of the 269 patients of the studied cohort; B) Boxplot representing albumin concentration values of the total cohort; C) Boxplot representing TT concentration values of the total cohort; D) Boxplot representing SHBG concentration values of the total cohort; E) Boxplot representing BT concentration results obtained in RIA of the total cohort; F) Boxplot representing BT concentration values obtained by calculation of the total cohort.
Comparison between BT calculated values and BT values obtained in RIA
Total cohort: For all patients (men and women), whose BT values obtained in RIA are >9 (in order to be comparable), according to the Pearson correlation test, we obtain a correlation coefficient of 0.94 (alpha=5%, p<0.001; values strictly lower than 9 have been excluded). The Wilcoxon test of the signed ranks shows a significant difference between BT obtained in RIA and calculated BT values (p<0.0001, alpha=5%; values strictly lower than 9 have been excluded). The Kolmogorov-Smirnov test, for its part, attests significantly comparable results between the BT obtained in RIA and calculated BT results (p=0.056, alpha=5%; values strictly lower than 9 have been excluded) (Figure 2).
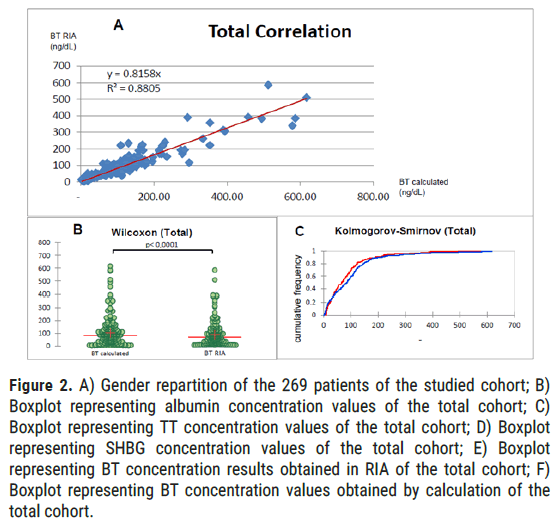
Figure 2: A) Gender repartition of the 269 patients of the studied cohort; B) Boxplot representing albumin concentration values of the total cohort; C) Boxplot representing TT concentration values of the total cohort; D) Boxplot representing SHBG concentration values of the total cohort; E) Boxplot representing BT concentration results obtained in RIA of the total cohort; F) Boxplot representing BT concentration values obtained by calculation of the total cohort.
Women: For BT results below 9 ng/dL obtained in RIA, that is to say 48 women out of the 80 of all the women in our sample (60%), all the calculated BT values are also less than 9 nmol/L, which is perfectly consistent. Wilcoxon test of the signed ranks shows a significant difference between BT obtained in RIA and the calculated BT values (p<0.0001, alpha=5%; values strictly lower than 9 have been excluded). For the entire female population of our sample, we find a modest correlation coefficient evaluated at 0.56 (alpha=5%, p<0.001) (Figure 3).
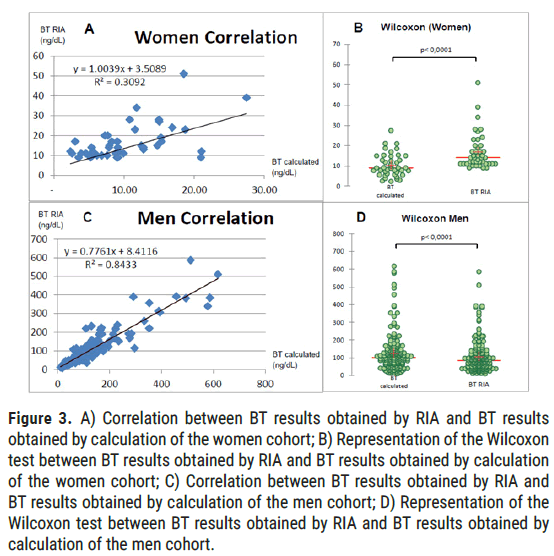
Figure 3: A) Correlation between BT results obtained by RIA and BT results obtained by calculation of the women cohort; B) Representation of the Wilcoxon test between BT results obtained by RIA and BT results obtained by calculation of the women cohort; C) Correlation between BT results obtained by RIA and BT results obtained by calculation of the men cohort; D) Representation of the Wilcoxon test between BT results obtained by RIA and BT results obtained by calculation of the men cohort.
Men: For men, according to Pearson's correlation test, we obtain a correlation coefficient of 0.92 (alpha=5%, p<0.001; values strictly lower than 9 have been excluded). Wilcoxon test of the signed ranks shows a significant difference between BT obtained in RIA and calculated BT results (p<0.0001, alpha=5%; values strictly lower than 9 have been excluded).
Men by age: For men aged less than 50 years (50 years included), i.e., 91 patients (48%), we obtain a correlation coefficient of 0.90 (alpha=5%, p<0.001). For men aged over 50 (50 years excluded), i.e., 98 patients (52%), we obtain a correlation coefficient of 0.95 (alpha=5%, p<0.001) (Figure 4).
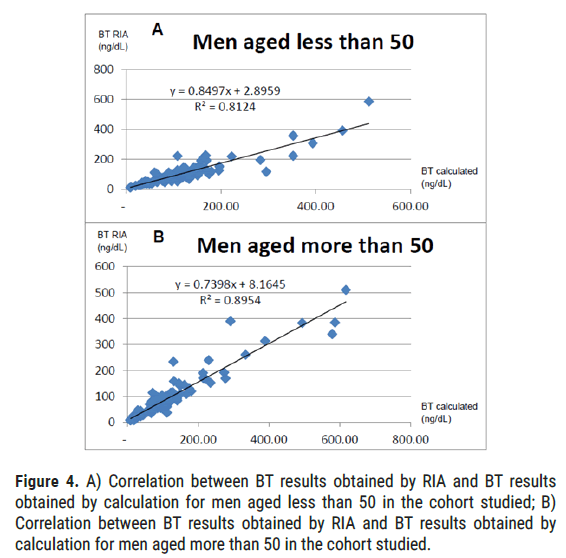
Figure 4: A) Correlation between BT results obtained by RIA and BT results obtained by calculation for men aged less than 50 in the cohort studied; B) Correlation between BT results obtained by RIA and BT results obtained by calculation for men aged more than 50 in the cohort studied.
Men by TT values: For normal TT values (from 188 to 684 ng/dL), we obtain a correlation coefficient of 0.78 (alpha=5%, p<0.001). These normal TT values represent 123 patients (65.1%). For low values of TT (<188 ng/ dL), we obtain a correlation coefficient of 0.94 (alpha=5%, p<0.001). These low TT values represent 49 patients (25.9%). For high values of TT (>684 ng/dL), we obtain a correlation coefficient of 0.84 (alpha=5%, p<0.001). These high TT values represent 17 patients (9.0%) (Figure 5).
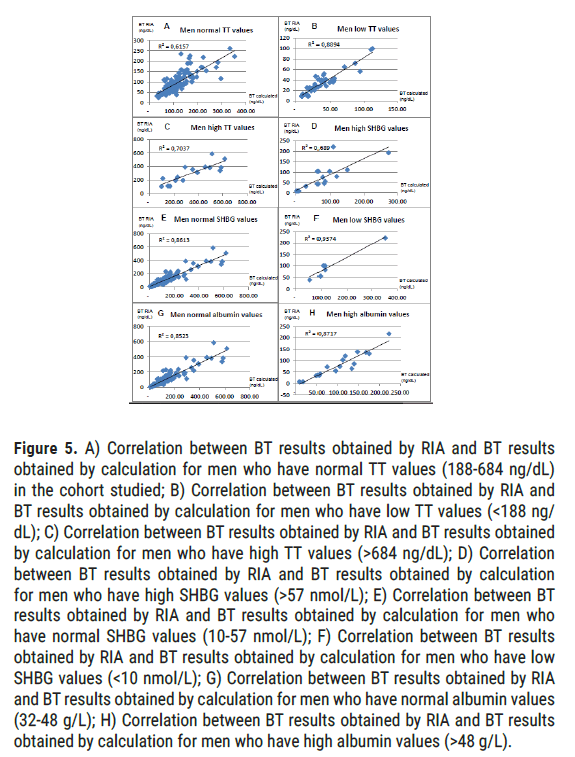
Figure 5: A) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have normal TT values (188-684 ng/dL) in the cohort studied; B) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have low TT values (<188 ng/ dL); C) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have high TT values (>684 ng/dL); D) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have high SHBG values (>57 nmol/L); E) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have normal SHBG values (10-57 nmol/L); F) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have low SHBG values (<10 nmol/L); G) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have normal albumin values (32-48 g/L); H) Correlation between BT results obtained by RIA and BT results obtained by calculation for men who have high albumin values (>48 g/L).
Men by SHBG values: For normal SHBG values (from 10 to 57 nmol/L), we obtain a correlation coefficient of 0.93 (alpha=5%, p<0.001). These normal values of SHBG represent 164 patients (86.8%). For low values of SHBG (<10 nmol/L), we obtain a correlation coefficient of 0.97 (alpha=5%, p<0.001). These low SHBG values represent 7 patients (3.7%). For high values of SHBG (>57 nmol/L), we obtain a correlation coefficient of 0.83 (alpha=5%, p<0.001). These high values of SHBG represent 18 patients (9.5%).
Men by albumin values: For normal albumin values (from 32 to 48 g/L), we obtain a correlation coefficient of 0.83 (alpha=5%, p<0.001). These normal albumin values represent 169 patients (89.4%). For low albumin values (<32 g/L), we obtain a correlation coefficient of 0.93 (alpha=5%, p<0.001). Note that these low albumin values represent only 3 patients (1.6%). For high albumin values (>48 g/L), we obtain a correlation coefficient of 0.93 (alpha=5%, p<0.001). These high albumin values represent 17 patients (9.0%).
For the entire population studied (men and women combined), Wilcoxon test shows a significant difference (p<0.0001, alpha=5%) between the values of BT calculated and BT obtained in RIA. Conversely, the Kolmogorov-Smirnov test shows that there is no significant difference between calculated BT and measured BT values. This testifies well to values being of the same order of magnitude. The correlation of the total cohort is excellent with a correlation coefficient established at 0.94 (p<0.001, alpha=5%). For men, this correlation coefficient is almost similar to 0.92(p<0.001, alpha=5%). On the other hand, for women, we obtained a very modest correlation coefficient of 0.56 (alpha=5%, p<0.001), this being mainly due to the fact that female population presents very low BT values, therefore much more sensitive to variations. At the same time, it should be remembered that this does not pose any problems in practice, the estimate of TT being sufficient in women. In men, the most relevant population in terms of application, it is interesting to note that calculated BT is very effective, and especially more in certain subgroups. Indeed, the calculated BT is closer to values obtained in RIA for men aged over 50 years old (r=0.95 for those over 50 vs. 0.90 for those under 50); for low (r=0.97) or normal (r=0.93) SHBG values rather than for high values (r=0.83); for low TT values (r=0.94 vs. 0.84 for high values and 0.78 for normal values), this is a very good thing, since it is precisely in this case that an estimate of BT is requested. Finally, it should be noted that whatever the value of the albumin, the correlation remains excellent (r=0.92; alpha=5%, p<0.001), thus making its application possible in severe states of undernutrition, which is often the case in the elderly population.
The strengths of our prospective study carried out over 2 years are numerous. In fact, this is based on a sample of 269 people including 189 men, which makes it a very large sample size for the interpretation of the results. We have a wide variety of patients from 18 to 87 years old with a very homogeneous spread. The same goes for the values of TT, BT, SHBG and albumin which are distributed in a very varied way. It should be noted that the values of BT that we find are mostly low, which is very interesting in practice since it is precisely in the event of suspicion of hypogonadism that this estimate represents the greatest interest in terms of extrapolation and interpretation as to the relevance of the results. Our large number of patients allowed us to identify 13 subgroups, these being large enough to be analyzed. In addition, the fact that we worked on paired samples is a major advantage in order to be able to compare with reliability the results of the two methods studied. Finally, for the calculated BT, we used the best calculation method available for the time being, namely the calculation optimized by Giton et al. of the Vermeulen equation [4]. Another small advantage of the calculation (provided of course that it gives a satisfactory result which seems to be the case) is that it is able to provide results lower than 9 ng/mL therefore more precise than the results determined by RIA which are in this case made less than 9 ng/mL (this therefore seems notably advantageous for the female population). Finally, it is advisable to insist on a big strong point of the calculation compared to the assay: it is that this assay (whether carried out in RIA or not), requires for the great majority of the laboratories an external sending (because of the absence of suitable automatons), thus generating additional costs, the necessity of an additional tube of blood, and a longer result delivery time. Our study, however, has some weak points. First of all, it should be noted that this is a single-center study, which only looked at patients from the Amiens University Hospital. It should also be noted that some of our subgroups, although small in number, have a relatively small population, which makes it a delicate interpretation. Last but not least, remember that RIA is not the gold standard for BT assay and is known to underestimate actual real BT values.
As a conclusion, we can say that the estimate of BT according to the optimized calculation of the Vermeulen equation by Giton et al. is highly recommendable in practice. However, it should be borne in mind that the results provided by this calculation are much less precise in women and that the performance of this calculation in men is a little lower for normal or even high TT values (few interests of the estimate of BT in this case) as well as for the high values of SHBG. By way of opening, we can therefore say that it would be extremely interesting to compare the calculated BT values with those obtained with the reference technique, namely ultrafiltration; and to benefit from an even larger population, so as to have even better supplied subgroups.
Citation: Philis Y, et al. Comparison Between Calculated Bioavailable Testosterone Level with Radio-Immunoassay Result. J Biol Today's World, 2021, 10(4), 001-005.
Received: 01-Jun-2021 Published: 22-Jun-2021, DOI: 10.35248/2322-3308.21.10.011
Copyright: © 2021 Philis Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.