Research Article - (2021) Volume 10, Issue 2
Aging has been targeted by dietary manipulation, drugs and antioxidants, to increase lifespan promotion of healthy aging. Pharmaceuticals, nutraceuticals and diets have been studied in various aging therapies with the ability to extend life span and postpone disease and dysfunction in animals. Starting from the metabolic theory of aging and the use of aging-mediator agent (D-galactose), this work was undertaken to demonstrate the possible role of Caloric Restriction (CR), Vitamin C (Vit C) and Metformin (Met) either alone or in combination to delay the aging process and promotion of health span and life extension in male albino rats; shedding the light on the cellular and molecular mechanisms underlying anti-age-related changes. Aging was induced by subcutaneous injection of D-galactose (D-gal) (100 mg/kg/d for 8 weeks). Liver samples were subjected to and relative mRNA and protein expression assessments. Additionally, the levels of serotonergic and dopaminergic neurotransmitters were estimated in the brain tissue. Histopathological examination of both liver and brain tissue were investigated as well. Aging (p53, Nrf-2, AKT, FoxO3 and JNK), apoptotic (Bax gene expression) and antiapoptotic (Bcl-2 gene expression), inflammatory (NF-κB and Cystatin C activity) and oxidative stress (MDA, GSH and CAT) markers were determined. D-Gal caused oxidative alterations of the liver observed via upregulation of aging, apoptotic, and inflammatory markers and downregulated the anti-apoptotic, antioxidant markers. Also, impairment in the neurotransmitters was observed. However, the combination between CR and Vit C and Met modulated the alteration in the parameters. Therefore, it can be concluded that the combination of CR and Vit C and Met were suitable for preventing some associated problems that could be used to maintain human health.
Aging • Caloric restriction • Vitamin C • Metformin • Apoptosis • Inflammatory biomarkers
The aging population and aging-related diseases have drawn great attention worldwide due to population increases and lifespan is extended. Aging is a biological phenomenon that raises morbidity and death rates as a progressive degeneration of physiological functions [1]. In addition, fatigue is easily seen as aging in both men and women, the incidence of fatigue reached its peak level for both men and women when individuals reached the age of 90 years [2]. There are several hypotheses on the mechanisms of changes associated with age. Currently, modern biological hypotheses of human aging fall into two major categories [3].
The theories that are programmed suggest that aging follows a biological schedule. The theories of harm or error emphasize environmental attacks on living organisms that inflict accumulated damage as the cause of aging at different stages. Aging in humans is associated with a significantly higher predisposition to several diseases, including cancer, type 2 diabetes, neurodegeneration, cardiovascular disease, muscle mass loss (sarcopenia), leading to increased morbidity and mortality [4]. Otherwise, aging is linked to regulatory pathways being dysregulated. Aging, for example, disturbs the balance between pro-and anti-inflammatory components, promotes chronic inflammation and can also impair other important pathways [5].
Aging has been targeted by dietary manipulation, drugs and antioxidants, to increase lifespan, promotion of healthy aging. The National Institute of Aging has studied numerous treatments, including pharmaceuticals, nutraceuticals and diets, with the ability to prolong life spans and postpone disease and dysfunction in animals. The beneficial effect of lifestyle on ageing met these rigorous requirements from a pharmaceutical point of view: fasting regimens, calorie restriction, exercise and the use of low-molecular-weight compounds, like Metformin [6].
In particular, Calorie Restriction (CR) has been demonstrated to be an emerging nutritional intervention that stimulates the anti-aging mechanisms in the body. In addition to lifespan, CR has also been reported to delay or prevent the occurrence of many chronic diseases in a variety of animals and human. Beneficial effects of CR on outcomes such as immune function, motor coordination, and resistance to sarcopenia have recently been reported [6]. Starting from the metabolic theory of aging and the use of aging mediator agent (D-galactose), this work was undertaken to demonstrate the possible role of CR, vitamin C and Metformin either alone or in combination to delay the aging process in male albino rats; shedding the light on the cellular and molecular mechanisms underlying anti-age-related changes.
Chemicals
D-galactose (D-Gal) was obtained from Sigma-Aldrich (St Louis, USA). Metformin (Met) (Metformin hydrochloride) was purchased from Merck Pharmaceuticals (Egypt) and Vitamin C (Vit C) was obtained from Universal Industries Pharmaceuticals Company, known as Unipharma (Egypt). All other reagents were commercially available and of analytical purity.
Animals
This study was conducted using sixty adult male albino rats (Sprague- Dawley strain) weighing 200-250 g obtained from Nile Company for Pharmaceuticals and Chemical Industries (Cairo, Egypt). Rats were maintained with free access to standard laboratory pellet chow and water ad libitum. Rats were kept in the laboratory under controlled conditions of temperature (27 ± 2°C) and humidity (60% ± 5%) with 12 h light/12 h dark cycles in well-ventilated cages. All experimental procedures were carried out according to “Guide for the Care and Use of Laboratory Animals.”
Experimental design
After an acclimatization period of 7 days, rats were randomly allocated and divided into equally-sized six groups of ten animals each.
Normal group (group I): Rats were received a basal diet for one month.
D-Gal group (group II): Rats injected subcutaneously by D-Gal once a day (300 mg/kg b.wt dissolved in physiological saline) for 6 weeks [7].
Met group (group III): Animals have received Met orally at a dose of 150 mg/kg/day concurrently injected subcutaneously by D-Gal as in group II for 6 weeks [8].
Vit C group (group IV): Rats were received Vit C orally at a dose of (150 mg/kg/day) concurrently injected subcutaneously with D-Gal as in group II then for 6 weeks [9].
CR group (group V): Rats fed with a restricted diet (30% off) concurrently injected with D-Gal as in group II for 6 weeks.
Met+CR+Vit C group (group VI): Rats fed with restricted diet (30% off) concurrently treated with the combination of Met as group III and Vit C as in group IV and injected with D-Gal injection as in group II.
At the end of the experiment, rats fasted overnight. Blood samples were withdrawn from the heart of each animal, under light anesthesia by urethane. Blood was allowed to coagulate and was then centrifuged at 3000 rpm for 15 min. Immediately after blood sampling, animals were sacrificed by cervical dislocation; liver and brain tissue were rapidly removed, washed in ice-cold saline, plotted against dry and weighed. The left lobe of each liver and brain tissues were dissected and placed in 10% formalin prepared in phosphate-buffered saline to be used for histopathological examination. A weighed part of each liver and brain were homogenized with ice-cooled phosphate buffer saline to prepare 20% w/v were homogenate. The homogenate was then centrifuged at 4000 rpm for 5 min at 4°C using a cooling centrifuge to remove cell debris. The aliquots were then kept at -80°C till the day of analysis.
Biochemical investigations
Determination of oxidative stress markers in liver tissue homogenate: Lipid peroxidative products were measured using the thiobarbituric acid test for Malondialdehyde (MDA), as described by Satoh [10]. Reduced Glutathione (GSH) contents were measured according to the method of Ahmed et al., while the activity of catalase (CAT) was determined according to Aebi [11,12].
Determination of neurotransmitters in the brain tissue homogenate: Serum level of determination of neurotransmitters in the brain tissue including; norepinephrine, dopamine, and serotonin (5-Hydroxy Tryptamine (5-HT) were made by a fluorometric method according to the method of Ciarolone [13].
Determination of nuclear factor-κB (NF-κB) levels in liver tissue and cystatin-C in serum: Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) level was measured in liver tissue using ELISA kit purchased from MyBiosource, Inc. (USA), catalog no. MBS764450 and cystatin-C level in serum was determined by using ELISA kit purchased from Abcam (UK) catalog no. ab201281.
Determination of mRNA gene expression of the tumor suppressor gene (p53), Bcl-2-associated X protein (Bax) and B-cell lymphoma 2 (Bcl-2) by quantitative real-time PCR (qRT-PCR) in liver tissues
Isolation of RNA and reverse transcription: The change in mRNA expression of p53, Bax, and Bcl-2 was examined. Using the manufacturer’s instructions of TRIzol reagent (Life Technologies, USA), total RNA was isolated from 30 mg liver tissue. 1% agarose gel electrophoresis stained with bromide of ethidium was used to confirm the integrity of RNA. The synthesis of the first strand complementary DNA (cDNA) was done with reverse transcriptase (Invitrogen) using 1μg of total RNA as the template according to the manufacturer's protocol.
Quantitative real-time Polymerase Chain Reaction (qPCR): RT-PCRs were performed in a thermal cycler step one plus (Applied Biosystems, USA) using Sequence Detection Software (PE Biosystems, CA). The oligonucleotides used in these experiments are listed in Table 1. The reaction mixture of total volume 25 μl consisted of 2X SYBR Green PCR Master Mix (Applied Biosystems), 900 nM of each primer and 2 μL of cDNA. PCR thermal-cycling conditions included an initial step at 95°C for 5 min; 40 cycles at 95°C for 20 s, 60°C for 30 s, and 72°C for 20 s. Relative expression of P53, Bax, and Bcl-2 mRNA was calculated using the comparative Ct method according to Pfaffl [14]. Calculations were performed by calculating the values of Δ cycle threshold (ΔCt) by normalizing the average Ct value of each treatment compared to the endogenous control β-actin.
| Gene | Primer sequence |
|---|---|
| Bcl-2 | F: 5′ATCGCTCTGTGGATGACTGAGTAC3′ R: 5′AGAGACAGCCAGGAGAAATCAAAC3′ |
| Bax | F: 5′AGGGTGGCTGGGAAGGC3′ R: 5′TGAGCGAGGCGGTGAGG3′ |
| P53 | F: CCAAACTGCTAGCTCCCATC R: CTTCTTGGTCTTCGGGTAGC |
| β-actin | F: 5′CCAGGCTGGATTGCAGTT3′ R: 5′GATCACGAGGTCAGGAGATG3 |
Table 1: List of primers used in PCR.
Western immunoblotting analysis of Forkhead box O3 (FoxO3), c-Jun NH2-terminal Kinase (JNK) JNK, protein kinase B (Akt) and Nuclear factor erythroid-2 Related Factor 2 (Nrf2) proteins in liver tissue homogenate: Liver tissue proteins were extracted using TRIzol reagent and protein concentration was quantified according to Bradford [15]. 20 μg of protein per lane were separated with 10% SDS-PAGE and transferred onto PVDA membranes. Membranes were incubated at room temperature for 2 h with blocking solution (5% nonfat dried milk in 10 mM TrisCl, pH 7.5, 100 mM NaCl, and 0.1% Tween 20), then incubated overnight at 4°C with primary antibodies toward FoxO3, JNK, Akt and Nrf2 proteins with β-actin as control. After washing three times in 10 mM Tris-Cl, pH 7.5, 100 mM NaCl, and 0.1% Tween 20, the membrane was incubated with the secondary monoclonal antibody conjugated to horseradish peroxidase at room temperature for 2 h, and then membranes were washed four times with the same washing buffer. The membrane was developed and visualized by chemiluminescence using Amersham detection kit according to manufacturer’s protocols, then exposed to X-ray film. Primary and secondary antibodies were purchased from Cell Signaling Technologies, USA. Quantification of FoxO3, JNK, Akt and Nrf2 proteins were performed using scanning laser densitometer (Biomed Instrument Inc., USA).
Histopathological study
Specimens from liver and brain of all examined groups were washed, dehydrated in ascending grades of ethyl alcohol, cleared in xylene and embedded in paraffin wax. Sections of 5-6 μm in thickness were cut out, deparaffinized and stained with Hematoxylin and Eosin (H and E) for examination under the light microscope [16].
Statistical analysis
The SPSS (version 20) was used in data analyses. Data were analyzed with one-way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test (DMRT). A value of p<0.05 was considered to indicate a significant difference between groups [17]. The data were expressed as mean ± standard error (SE). P values <0.05 were considered statistically significant.
Biochemical studies
CR, Met and Vit C inhibited D-Gal-induced oxidative stress by elevating antioxidants in rats: As oxidative stress plays an important role in the aging process and stimulating free radical production is reported as an important mechanism underlying the D-Gal induced aging, we evaluated the most recognized oxidative damage product, MDA. D-Gal treatment greatly increased the MDA level in liver tissue homogenate compared with the normal group. Rats fed with restricted diet lonely and/ or combined with Met and Vit C supplementation significantly inhibited the D-Gal increased MDA, suggesting that lonely or in combination CR and Met and Vit C attenuated the aging by inhibiting oxidative stress in respect with the G-Gal group (Table 2). However, D-Gal injection decreased the level of GSH and CAT activity in the liver tissue, on which CR, Met and Vit C lonely or in combination showed effective protection, suggesting that CR, Met and Vit C lonely or in combination may inhibit D-Gal-induced oxidative stress by elevating these antioxidants (Table 2).
| Parameters/ Groups | Normal | D-Gal | Met | Vitamin C | CR | Met+CR+Vit C |
|---|---|---|---|---|---|---|
| MDA (µmol/mg tissue) |
44.9 ± 1.2 | 126.0 ± 6.6 | 83.4 ± 1.3 | 81.6 ± 2.1 | 78.8 ± 3.2 | 55.8 ± 7.1 |
| GSH (µmol/mg tissue) |
95.4 ± 2.9 | 39.1 ± 6.7 | 65.6 ± 2.2 | 62.4 ± 7.3 | 63.9 ± 2.6 | 81.8 ± 1.7 |
| CAT (U/g tissue) |
212.1 ± 1.4 | 106.9 ± 5.6 | 182.4 ± 7.2 | 188.9 ± 10.4b | 184.5 ± 11.3b | 200.2 ± 8.1b |
Data are expressed as mean ± SE; n=10. Intergroup comparisons were performed by ANOVA. Data are expressed as mean ± SD (n=10). Values sharing different letters are significantly different (P<0.05).
Table 2: Effects of CR, Met and Vitamin C lonely or in combination on oxidative stress biomarkers in liver tissue of D-Gal-induced aging rats.
CR, Met and Vit C modulated biogenic amines in D-Gal-induced alteration in serotonergic function: Effect of CR, Met and Vit C lonely or in combination on biogenic amines (serotonin, dopamine and norepinephrine) concentration in brain of D-Gal group was determined. Data in Table 3 showed that the injection of D-Gal resulted in severe alterations in levels of neurotransmitters contents (serotonin, dopamine and norepinephrine) in the brain of rats. Rats fed with restricted diet lonely and/or combined with Met or Vit C supplementation significantly reduced the alteration in levels of neurotransmitters contents in respect to the D-Gal injected group.
| Parameters/Groups | Normal | D-Gal | Met | Vitamin C | CR | Met+CR+Vit C |
|---|---|---|---|---|---|---|
| Serotonine (ng/mg tissue) |
145.9 ± 2.3 | 60.8 ± 2.4 | 106.9 ± 5.4 | 109.8 ± 1.2 | 108.1 ± 1.8 | 122.1 ± 1.5 |
| Dopamine (ng/mg tissue) |
65.9 ± 3.4 | 24.7 ± 4.6 | 41.5 ± 3.7 | 35.4 ± 0.9 | 44.5 ± 2.4 | 56.7 ± 1.6 |
| Norepinephrine (ng/mg tissue) |
14.2 ± 1.4 | 37.4 ± 1.8 | 18.4 ± 1.2 | 19.2 ± 2.4 | 26.6 ± 2.4 | 28.4 ± 0.8 |
Data are expressed as mean ± SE; n=10. Intergroup comparisons were performed by ANOVA. Data are expressed as mean ± SD (n=10). Values sharing different letters are significantly different (P<0.05).
Table 3: Effect of CR or Met or Vit C lonely or in combination on serotonergic and dopaminergic functions in brain of D-Gal-induced aging rats.
Effects of CR, Met and Vit C on related proteins in the cystatin C and NF-κB pathways: Detection of the levels of aging-related protein factors is shown in Figure 1, it is shown that long-term administration of D-gal led to a marked increase of cystatin C in serum and NF-κB in liver tissue of rats compared with the normal healthy group. After treatment with Met, Vit C and/or combination with CR reduce the levels of cystatin C and NF-κB compared with the D-Gal group, implying that CR or Met or Vit C lonely or in combination exerted theirs anti-aging effects through the cystatin C and NF-κB signaling pathways.
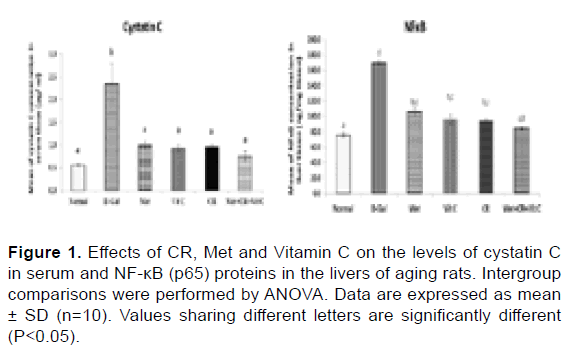
Figure 1: Effects of CR, Met and Vitamin C on the levels of cystatin C in serum and NF-κB (p65) proteins in the livers of aging rats. Intergroup comparisons were performed by ANOVA. Data are expressed as mean ± SD (n=10). Values sharing different letters are significantly different (P<0.05).
Effects of CR, Met and Vit C on p53/Bcl-2/Bax signaling pathwayrelated genes expression in liver tissue: To better study the effects of Met or Vit C and/or combination with CR on the p53/Bcl-2/Bax signaling pathway, the key genes (p53, Bcl-2, Bax) related to the signaling pathway in mRNA levels were detected. D-Gal down-regulated Bcl-2 expression and up-regulated Bax and p53 expression accompanied by increased ratio between Bax/Bcl-2 compared with the control group (Figure 2). However, treatment with Met, Vit C and/or combination with CR significantly suppressed the mRNAs expressions of p53 and Bax, concomitant with overexpression in the gene of Bcl-2 in liver tissue in respect with the D-Gal injected group (Figure 2).
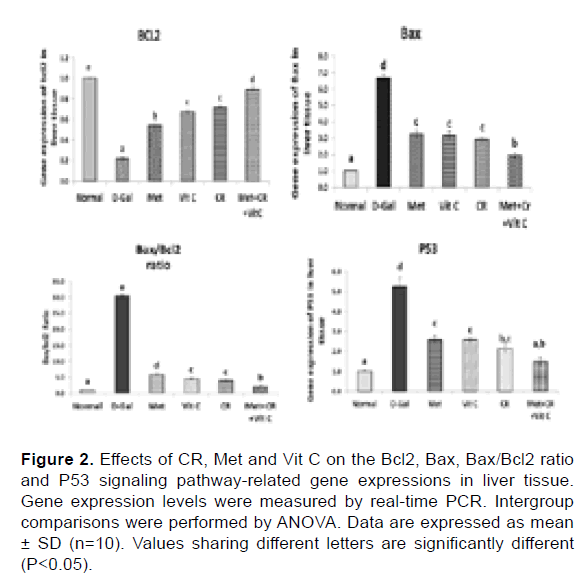
Figure 2: Effects of CR, Met and Vit C on the Bcl2, Bax, Bax/Bcl2 ratio and P53 signaling pathway-related gene expressions in liver tissue. Gene expression levels were measured by real-time PCR. Intergroup comparisons were performed by ANOVA. Data are expressed as mean ± SD (n=10). Values sharing different letters are significantly different (P<0.05).
Effects of CR, Met and Vit C on survival pathways: We further examined whether the survival proteins’ expression increased with Met, Vit C and/or combination with CR in the aging rat liver. The protein levels of Nrf-2, Akt and FOXO-3 were significantly decreased accompanied with significant overexpression in the level of JNK in the aging group, but after being treated with Met, Vit C and/or combination with CR, the expression of Nrf-2, AKT and FOXO-3 were increased significantly compared with those in the aging group. Whereas, the protein level of JNK was significantly reduced (Figure 3).
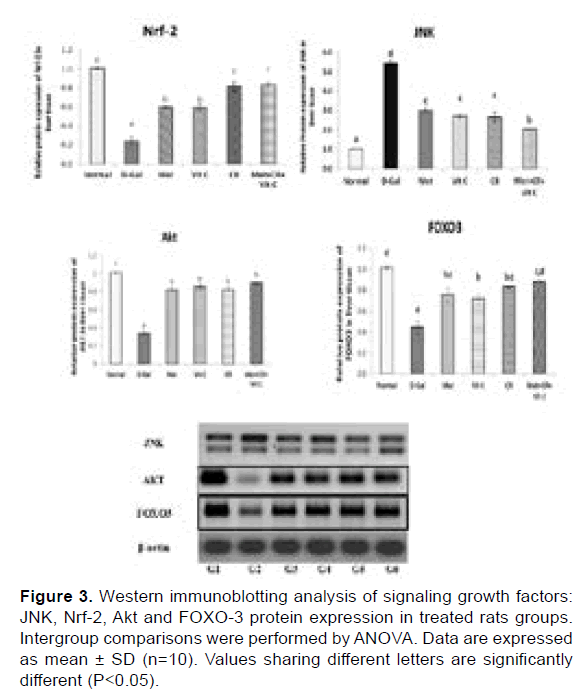
Figure 3: Western immunoblotting analysis of signaling growth factors: JNK, Nrf-2, Akt and FOXO-3 protein expression in treated rats groups. Intergroup comparisons were performed by ANOVA. Data are expressed as mean ± SD (n=10). Values sharing different letters are significantly different (P<0.05).
Histopathological findings
Microscopic examination of the liver tissue from the control group revealed diffuse glycogen accumulation in the hepatic parenchyma and swollen hepatocytes with a mosaic histological pattern and compression of the hepatic sinusoids (Figure 4A). In the D-Gal group, the liver showed multifocal areas of lytic necrosis, which appeared as mononuclear inflammatory cell infiltration associated with eosinophilic karyorrhectic debris. Multifocally, portal areas and hepatocytes were infiltrated with inflammatory cells. Some examined sections showed macro and microvesicular steatosis of the periportal hepatocytes with more binucleated cells. Meanwhile, some individuals showed severely congested hepatic sinusoids (Figure 4B). In the Met group, few mononuclear inflammatory cell infiltrations were detected in the portal area in some instances with portal edema, and condensed nuclear chromatin in the adjacent hepatocytes. Alternatively, some other hepatic lobules of the same group showed mild vacuolar degeneration (Figure 4C). In the Vit C group, the hepatocytes displayed mild vacuolation with detection of mitosis in hepatic cells. Mitochondrial hypertrophic hepatocytes were also frequently detected in the hepatic parenchyma with microvesicular steatosis (Figure 4D). In the CR group, the histological pattern presented a mosaic glycogen infiltration similar to the control group, however, excessive accumulation of glycogen was observed in some sections with severe narrowing of hepatic sinusoids (Figure 4E). Concerning the Met+VitC+CR group, multifocal areas of hepatitis were observed which were characterized by mononuclear cell aggregation with surrounding hepatocytes showed dark nuclei. Expansion of the portal area was detected with heavy nonnuclear inflammatory cells and oval cell hyperplasia. Thickening of the portal blood vessels with blebbing of endothelial lining was also seen (Figure 4F).
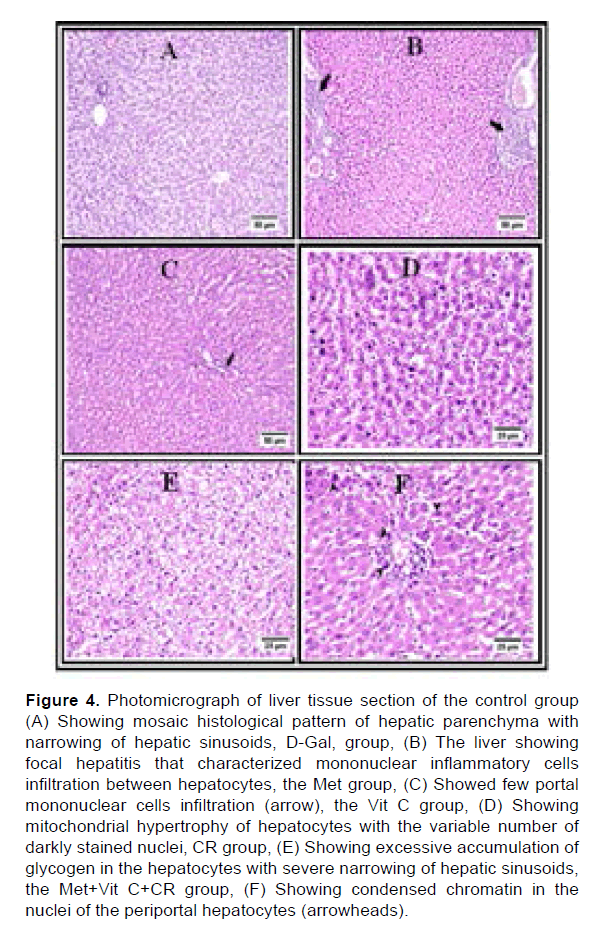
Figure 4: Photomicrograph of liver tissue section of the control group (A) Showing mosaic histological pattern of hepatic parenchyma with narrowing of hepatic sinusoids, D-Gal, group, (B) The liver showing focal hepatitis that characterized mononuclear inflammatory cells infiltration between hepatocytes, the Met group, (C) Showed few portal mononuclear cells infiltration (arrow), the Vit C group, (D) Showing mitochondrial hypertrophy of hepatocytes with the variable number of darkly stained nuclei, CR group, (E) Showing excessive accumulation of glycogen in the hepatocytes with severe narrowing of hepatic sinusoids, the Met+Vit C+CR group, (F) Showing condensed chromatin in the nuclei of the periportal hepatocytes (arrowheads).
Microscopic examination of the brain tissue from the control group revealed apparently normal histological structure in most examined sections with normal pyramidal cells and different types of neuralgia cells were scattered inside the neuropil matrix, meanwhile, some examined tissue showed mild demyelination in the striatum. Few dark shrunken neurons with perineuronal edema were observed in the cerebral cortex. The hippocampus displayed apparently normal well-organized neurons in the different Cornu Ammonis regions (CA) and the Dentate Gyrus (DG) (Figure 5A). In the D-Gal group, there is severe congestion with perivascular hemorrhage in the Virchow Robin space of cerebral cortex vessels, also thickening of the vascular wall was detected with adjacent diffuse gliosis. The striatum revealed severe demyelination with considerable hemorrhagic areas (Figure 5B). The hippocampus exhibited severe necrotic neurons among different CA and DG regions. Moreover, the meninges showed severe hemorrhage (Figure 5B). In the Met group, it was found that perineuronal edema was increased compared to the control group and was markedly observed in the striatum. A focal area of hemorrhage was detected in the neuropil matrix of the cerebral cortex. The examination of the hippocampus revealed apparently normal neurons in most examined sections, however, some CA regions showed dark shrunken neurons (Figure 5C). In the Vit C group, neuronal degeneration and neuronphagia were seen in the cerebral cortex. The striatum showed few necrotic piriform neurons with numerous scattered perineuronal edema. The hippocampus displayed decrease cell density in the CA regions with numerous scattered dark degenerated neurons in CA and DG regions. However, other individuals presented apparently well-organized neurons in the different hippocampus regions. Additionally, the brain stem showed multifocal hemorrhagic areas (Figure 5D). Referring to CR group, numerous vascular congestion was detected in the cerebral cortex. The striatum triggered excessive demyelination in some instances. Decreased cellular density was also noticed in the hippocampus region that was associated with dark degenerated neurons (Figure 5E). Various histopathological alterations were detected in the cerebral cortex of the Met+VitC+CR group with severe cortical congestion and diffuse gliosis with activated microglia cells. The hippocampus displayed numerous dark degenerated neurons (Figure 5F).
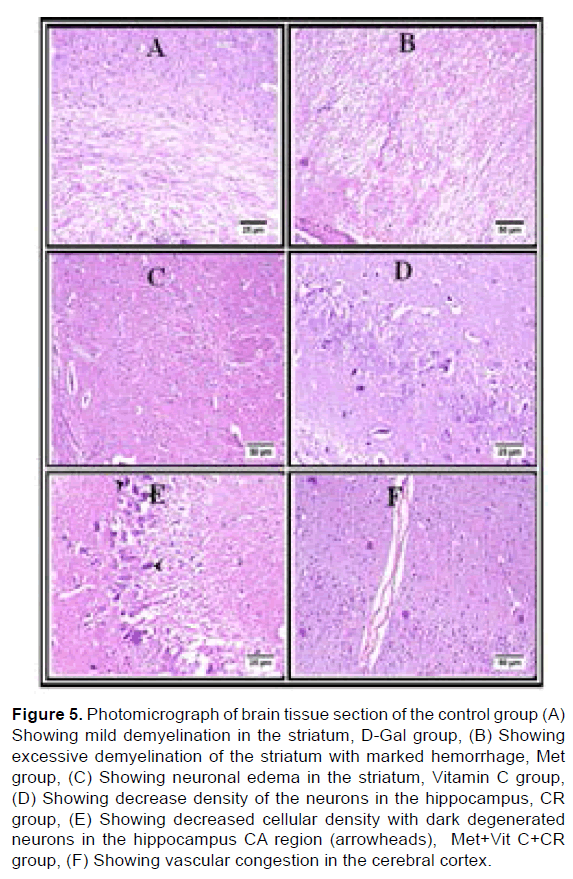
Figure 5: Photomicrograph of brain tissue section of the control group (A) Showing mild demyelination in the striatum, D-Gal group, (B) Showing excessive demyelination of the striatum with marked hemorrhage, Met group, (C) Showing neuronal edema in the striatum, Vitamin C group, (D) Showing decrease density of the neurons in the hippocampus, CR group, (E) Showing decreased cellular density with dark degenerated neurons in the hippocampus CA region (arrowheads), Met+Vit C+CR group, (F) Showing vascular congestion in the cerebral cortex.
Aging is a complicated multifactorial phenomenon, which means that multiple pathways need to be targeted to effectively prevent or delay aging [18]. Several molecular pathways are well known for affecting aging, but only a few have been successfully targeted through lifestyle changes such as caloric restriction or individual drug targeting, but either caloric restriction or individual drugs do not target all aging pathways individually. Therefore, combinations of caloric restriction and individual anti-aging drugs can effectively broadening the scope of aging targets. So, the current study tested the effect of a combination of caloric restriction and/ or Metformin and vitamin c against D-Gal-inducing aging in rats (Figure 6).
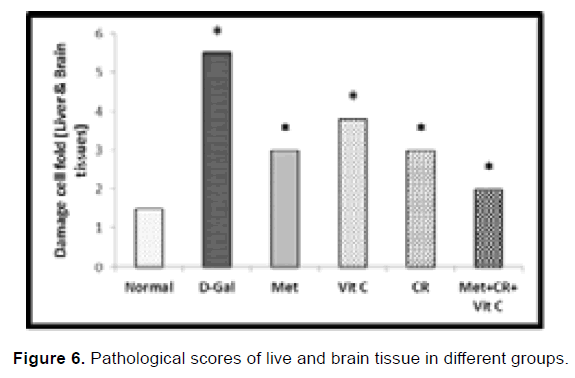
Figure 6: Pathological scores of live and brain tissue in different groups.
Accumulating evidence indicates that superfluous administration of D-Gal is correlated with increased oxidative stress, reduced antioxidants and increased apoptosis of the cells, which is strongly related to aging [19]. Our findings showed that a combination strategy between treatment with CR and Met and Vit C protected the liver and brain of rats against oxidative stress caused by D-galactose by controlling multiple intracellular and redox- sensitive signaling pathways. Nrf2 is an important transcription stress-response factor noted for its cytoprotective role. Nrf2 dissociates from Kelch-like ECH-associated protein 1 (Keap1) under oxidative stress conditions and translocates into the nucleus, inducing the enhancement of CAT phase II enzyme activity and GSH content and the anti-apoptotic protein Bcl-2 [20,21].
The growth factor Akt protein, which plays a crucial role in controlling cell survival and apoptosis, is affected by the nuclear translocation of Nrf2 [22]. The signal transduction of FoxO3 protein, associated with ageing and controlling the stress response, apoptosis, longevity and cell cycle, is also regulated by Akt [23]. In the generation of ROS, FoxO3 plays a key role and mediates the activation of antioxidant genes, such as GSH and CAT [24,25]. Western blotting analysis showed in the current study that D-Gal injection significantly reduced the levels of Nrf2, Akt and FOXO3, where CR and Met and Vit C supplementation increased the expression of proteins Nrf2, Akt and FoxO3 in the rat liver compared to the D-Gal group. These findings suggested that multiple antioxidants, including GSH and CAT, were enhanced by CR and Met and Vit C supplementation by activating Nrf2 and AKT and FOXO3, indicating that Akt and FoxO3 activities were inhibited by D-Gal treatment but retrieved by treatment with CR and Met and Vit C. Taken together the Akt/FoxO3 route is involved in the ageing process caused by D-Gal and has acted as another pathway for oxidative stress and apoptosis. Therefore, CR and Met, Vit C treatment may show protective effect via activating the Akt pathway and enhancing the expression of its downstream targets and/or factors such as FoxO3a and Nrf2.
It is estimated that over-expressed Bcl-2 is resistant to cell apoptosis, but by making a heterodimer with Bcl-2 and blocking its anti-apoptotic actions, Bax promotes cell death [26]. The Bax/Bcl-2 expression ratio is, therefore, considered as a central determinant of the fate of a cell. In our experiments, the D-Gal-induced ageing rats found an increased Bax/Bcl-2 expression ratio, which suggested d-Gal-induced cell apoptosis [19]. As anticipated, following CR and Met and Vit C therapy, a reduced Bax/Bcl-2 expression ratio was presented. The lower Bax/Bcl-2 ratio and decreased Bax protein led to decreased apoptosis, leading to oxidative stress and aging caused by D-Gal defense. Akt/FoxO3 could contribute to the antiapoptotic effect of CR and Met and Vit C treatments as a well-known apoptosis-relevant signaling pathway.
The elevated Akt protein expression described above verified this hypothesis. These data strongly support treatment with CR and Met and Vit C unregulated the Bcl-2/Bax ratio by activating the Akt pathway and therefore exerted its antioxidant role in aging mice induced by D-Gal. The p53 tumor suppressor pathways are typically the mechanisms involved in senescence [27]. Previous studies reported that p53 gene expression was up-regulated in D-Gal-treated rats. It has been suggested that p53 is phosphorylated by JNK protein through the inhibition of ubiquitin-mediated degradation of p53, thereby stabilizing the levels of p53. Several studies have shown that JNK phosphorylation of p53 is critically essential for the apoptotic pathway [28]. JNK may therefore stimulate the expression of pro-apoptotic genes and decrease the expression of pro-survival genes by many cell type and stimulus-specific transcription factors. Consistently, D-Gal increased ROS generation in the current study and further enabled P-JNK, which significantly increased the Bax/Bcl-2 ratio, likely by accelerating apoptosis and the aging process. Over-activated p53 can decrease FOXO3 expression and accelerate cell apoptosis induced by DNA damage. The expression of JNK protein was significantly reduced by CR and Met and Vit C supplements and the p53 gene expression was, therefore, downregulated. Increased FoxO3 expression by treatment with CR and Met and Vit C may form inverse feedback to inhibit p53 activity, which has contributed to cell survival and anti-aging. Current results might help to understand the different roles of the JNK/ p53/FoxO3 pathway in D-Gal-induced aging.
Meanwhile, chronic inflammation is an important manifestation of oxidative stress and a major contributor to aging and aging-related degenerative disease pathogenesis [29]. A major inflammatory pathway triggered by several external and internal stimuli, including elevated ROS, hypoxia, and genotoxic stress, is the NF-κB pathway [30]. In response to different stimuli, such as inflammation, infection and DNA damage-induced oxidative stress, NF-κB, a family of homo-and heterodimeric transcription factors, are involved in the homeostasis of various transcription genes. Elevated NF-κB has been reported in old age [31]. Similarly, Lu et al reported activated NF-κB in the D-Gal mouse model [32].
Furthermore, cystatin C was found to be capable of inducing the activity of NF-κB [33]. In this study we investigated D-Gal increased NF- κB and cystatin C levels, which caused other mediators of inflammation. Therapy with CR and Met and Vit C reverses D-Gal-induced elevated NF-κB activity.
The brain undergoes numerous functional and structural modifications during the normal aging process that affects the dendritic and synaptic connections, the circulation of the blood and metabolism of various neurotransmitters, resulting in impaired neurotransmission [34]. Neurotransmission is a highly complex mechanism assisted by continuous neurotransmitter cycling, and neurotransmitter concentration tests in the brain can provide a mirror image of neurotransmitter status [35]. D-Gal injection has affected the serotonergic and dopaminergic processes in many neurotransmitter systems. Major reductions in serotonin levels were observed in D-Gal rats in the brain, while treatment with CR and Met and Vit C significantly increased serotonin and dopamine levels in brain tissue. In D-Gal injected rats, however, norepinephrine levels were found to be increased, but decreased in the brain CR and Met and Vit C-treated rats. The activity of serotonin and dopamine rate-limiting enzymes is documented to be reduced during aging. Previous studies have shown a clear correlation between oxidative changes during aging and the reduced or low activity of serotonin and dopamine synthesizing enzymes [36]. Such increases in oxidation contributed to diminished brain dopaminergic and serotonergic functions and ultimately resulted in age-related psychiatric problems such as depression, anxiety and stress. Oxidative stress initiates apoptosis in nerve cells during aging, leading to cell death and neuronal failure [37]. Serotonergic and dopaminergic dysfunction is due to an increase in oxidative stress that initiates the brain's apoptotic process and as stated earlier, results in dopaminergic and serotonergic neuronal damage, ultimately resulting in decreased levels of neurotransmitters [38].
It can be inferred from the data obtained that D-Gal injection causes oxidative stress, multiple molecular changes, inflammatory cascade, pathological apoptosis, and natural aging-like neurological dysfunction. Caloric restriction or treatment with metformin or vitamin C improved aging results in rats on an individual basis, but the combination of rats had a greater protective effect than the caloric restriction or individual drugs alone. The protective effect of combination between CR and Met and Vit C involves regulating the JNK/ p53/FoxO3 pathway and enhancing the overall antioxidant capacity by targeting the key antioxidant transcription factors such as Nrf-2 and Akt proteins. Therefore, these findings in the rat suggest that the combination of caloric restriction, metformin and vitamin c may provide a more optimal approach than either therapy alone in the management of aging.
Funding
The authors did not receive support from any organization for the submitted work.
Financial or non-financial interests
The authors have no relevant financial or non-financial interests to disclose.
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval
All experimental procedures were carried out according to “Guide for the Care and Use of Laboratory Animals.”
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed. The first draft of the manuscript was written by Fatma SM Moawed and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Citation: Abozaid OAR, et al. Caloric Restriction Combined with Vitamin C and Metformin Regulates Inflammatory Mediators through the JNK/ p53/FoxO3 Pathway in Aged Rats. J Biol Today's World, 2021, 10(2), 001-007.
Received: 22-Jan-2021 Published: 12-Feb-2021, DOI: 10.35248/2322-3308.21.10.005
Copyright: © 2021 Abozaid OAR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.