Review Article - (2023) Volume 8, Issue 1
The death of David, BENNET (the first man to have been transplanted, on January 7, 2022, with a pig's heart), 2 months after the operation has revived the debate on animal organ transplants which will probably be a real alternative to transplants in a few years. allografts. Indeed, despite enormous efforts made over the last 50 years, the number of human organs available from deceased subjects for clinical transplantation purposes is very insufficient compared to the demand. For example, in the United States alone, there are currently over 120,000 patients waiting for transplants but only 30,000 transplants are performed each year using organs from deceased donors. However, transplant surgeons have liberalized their donor selection criteria, now sometimes using organs from so-called "high-risk", "marginal" or "extended criteria" deceased donors, which are organs from donors who would not have been used a few years ago. The first xenotransplantations were carried out in the 17th century. But it was at the beginning of the 20th century that their history, punctuated by multiple adventures, began. The detection of porcine virus in Bennett's heart is perhaps paradoxically good news for the future of xenotransplantation if it is the main cause of transplant failure. United Therapeutics companies and eGenesis plan to start clinical trials using pig organs within the next year or two.
Xeno-transplantation • Immune barriers • Lymphocytes • Oncogenesis
The death of David, BENNET (the first man to have been transplanted, on January 7, 2022, with a pig's heart ), 2 months after the operation has revived the debate on animal organ transplants which will probably be a real alternative to transplants in a few years. allografts. Indeed, despite enormous efforts made over the last 50 years, the number of human organs available from deceased subjects for clinical transplantation purposes is very insufficient compared to the demand. For example, in the United States alone, there are currently over 120,000 patients waiting for transplants but only 30,000 transplants are performed each year using organs from deceased donors [1]. However, transplant surgeons have liberalized their donor selection criteria, now sometimes using organs from so-called "high-risk", "marginal" or "extended criteria" deceased donors, which are organs from donors who would not have been used a few years ago.
The gap between the number of patients on transplant waiting lists Tand o solvthe e the ornumber gan shorof tage throrgans ee possibleavailable apprcontinues oaches hato vegrow emerin ged:all countries (Figure 1).
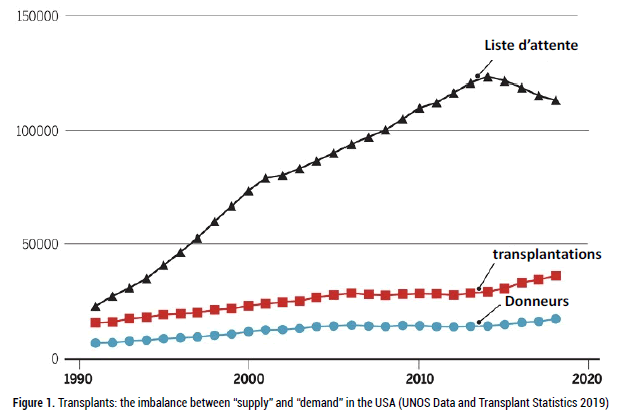
Figure 1: Transplants: the imbalance between “supply” and “demand” in the USA (UNOS Data and Transplant Statistics 2019)
• Increase in the number of donations from deceased individuals. But only a very small proportion of deaths make it possible to obtain transplantable organs (brain death and donations after cardiac arrest, which represent less than 1% of deaths), so the potential success of this approach is limited. The gap between the need for and the availability of organs is not narrowing, even in countries with "presumed consent" laws for the removal of organs from brain-dead people who have not specified their wishes regarding organ donation,
• Use of tissue "Bioengineering" techniques to create transplantable organs. This approach often uses stem or progenitor cells to reconstitute organs produced either by 3D printing or by decellularization then recellularization of human or animal organs. An organ from a deceased donor is treated with different solutions containing detergents or enzymes to remove all cells from the tissue, leaving only the extracellular matrix with the original structure and shape of the organ. The extracellular matrix is then seeded with cells taken from the patient. This technique would avoid immune system reactions and the risk of long-term rejection.
• Use of animal organs, or xeno-transplantation. It is conceivable to ensure a virtually unlimited supply of tissues and organs from lower mammalian species such as pigs. The field of xenotransplantation was initiated by a series of kidney transplants from chimpanzees to humans in the early 1960s. most suitable xenograft donor for several reasons, including organ size, availability, reproductive characteristics, and physiological similarities to humans. The main obstacle to the use of pork was the presence in primates, and in particular in humans, of a high titer of natural antibodies directed against porcine antigens, these antibodies leading to Hyperacute Rejection (HAR) of xenografts. However, with advances in gene-editing technology and better knowledge of the mechanism of immune rejection of porcine organs, transplantation of porcine organs to humans has become a realistic option, with, in January 2022, the first attempt heart transplantation from pigs to humans.
Leschimpanzees, as an endangered species, could not meet the growing need for organ transplants. The use of lower mammalian species had not been attempted until 1990 because such transplants tested in Non-Human Primates (NHPs) had been consistently rejected within minutes or hours, due to the high titer of natural antibodies present in all recipients Until 1980, clinical needs were met by donations from deceased persons, transplants in humans being still uncommon. It was the success of allogeneic transplants, thanks to the use of new immunosuppressive drugs at the end of the 1980s, limiting rejection reactions, which paradoxically led to a renewed interest in xeno-transplantation. The use of organs from de non-human primates autres than the chimpanzee (whose use had become banned) was plagued with issues of size, availability, viral pathogens and ethics. Nevertheless, several attempts to use baboon organs in humans have been made with patient survivals ranging from only 20 to 70 days. In the field of transplantation, all these difficulties have led to the search for another xenograft donor animal which is less problematic.
In the mid-1990s, it was finally the pig that appeared to be the most appropriate animal source of xenografts for the reasons mentioned in the introduction. The expected success of xeno-transplants has many advantages over allogeneic transplants, in particular almost unlimited availability and better quality control for both the function of the transplanted organs and the lower risk of the presence of infectious agents. Currently, elective allografts that are scheduled in advance are only possible for living donation (kidney, liver, etc.) but if xeno-transplantation becomes a reality, transplants will be elective operations (the donor animal being chosen in advance) that can be offered before serious complications of organ failure arise.
The first xeno-transplantations were carried out in the 17th century. But it was at the beginning of the 20th century that their history, punctuated by multiple adventures, began.
1682: A fragment of dog bone was used to repair the skull of a Russian nobleman who had been injured. This is the first animal tissue transplant on humans. The operation was considered a success, but the Russian church threatened the subject with excommunication, that -cihad the graft removed.
1906: Two kidney transplants were performed for the first time, at the bend of the elbow of two women with kidney failure. The French surgeon Mathieu Jaboulay used a porcine donor for one of the patients and a goat donor for the other. Both transplants failed and the patients died a few days after the operation.
1910: German physician Ernst Unger, after performing more than 100 animal kidney transplants all of which failed, transplanted both kidneys from a Borneo monkey into the groin of a 21-year-old female patient who suffered from kidney failure. The patient died of pulmonary edema on the second day after the operation. Unger concludes that there was a biochemical barrier between animals and humans that made transplants impossible.
1920: Serge Voronoff, a Russian doctor who emigrated to France, a pupil of Alexis Carrel, grafted tissue from monkey testicles onto elderly men (Figure 1). In 1930, more than five hundred men were treated in France by this “rejuvenation” technique. These experiments did not have the expected result which was an artificial increase in the effects of hormones in order to delay aging. Voronoff's numerous patients only owe their rejuvenation, as well as all the other benefits of the transplant, to a placebo effect (Figure 2).
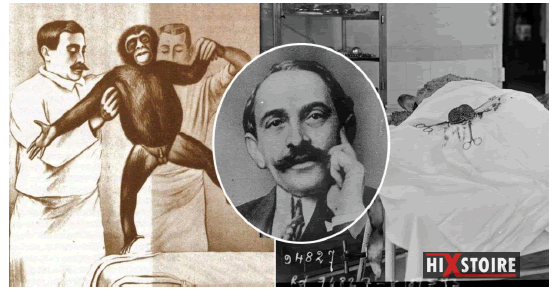
Figure 2: Serge VORONOFF, the surgeon who grafted monkey testicles to men to rejuvenate them and improve their libido.
1963-64: Baboon kidneys were transplanted into six patients by surgeon Thomas Starzl in Denver, USA. Patients survived between 19 and 98 days.
1963-64: Chimpanzee kidneys were transplanted by Tulane University surgeon Keith Reemstma, into 12 patients in New Orleans, USA. Most failed within two months, but one patient survived for nine months, returning to teaching without any sign of rejection; she succumbed to an ionic imbalance.
1964: James Hardy of the University of Mississippi in Jackson, USA attempted to transplant un a 68-year-old man with a chimpanzee heart, but the patient only survived two hours, the transplanted heart was too small to ensure adequate blood circulation in the recipient.
1969-1974: Three children received chimpanzee livers but only survived between 1 and 14 days.
1977: Christian Barnard, who succeeded in the first heart transplant (allogeneic transplant) in 1967, attempted in Capetown, South Africa, to transplant baboon and chimpanzee hearts as a temporary assist pump in a 25-year-old woman and a 60-year-old man whose heart was not working properly after heart surgery. But despite high doses of immunosuppressive drugs, the patients died after a few days.
1984: Baby Fae, a little girl, received a baboon's heart in California. Cyclosporine was used as an immunosuppressant but the infant survived only 20 days.
1992: Baboon liver transplants were performed at the University of Pittsburgh. One of the patients survived 71 days without rejection. A cocktail of four immunosuppressive drugs had been administered to this recipient who died of an infection.
1992: A pig liver was implanted alongside the patient's own liver until a human organ became available, but the patient died after 32 hours.
1993: A baboon bone marrow and kidney transplant was performed in Pittsburgh, USA, with a cocktail of immunosuppressive drugs s. However, the weakening of the patient's immune system caused his death from infection after 26 days.
1995: A patient, Jeff Getty received baboon immune cells to try to stop his AIDS. The patient's condition improved, although the transplanted cells were quickly eliminated.
1997: Fetal pig nerve cells were transplanted into patients with Parkinson's disease: they functioned for seven months in the patients' brains.
2021: After a long halt to xenotransplantation attempts, a kidney from a transgenic pig was experimentally transplanted into a brain-dead patient.
2022: For the first time, a transgenic pig heart transplant was performed on January 7, 2022, at the University of Maryland by Doctor Bartley Griffith. The patient died two months later, on March 8, 2022 (Table 1).
Table 1. Xeno-transplantations: chronology: rejection mechanisms and innovations.
| Before 1980 | 1980 - 1990 | 1990 - 2000 | 2000 - 2010 | 2010 - 2020 | After 2020 | |
|---|---|---|---|---|---|---|
| Organ survival | Minutes | Minutes to hours | days to weeks | Month | months to years | Permanent? |
| Type of rejection | Hyper-acute rejection | Delayed rejection | Mediated rejection by antibodies | Chronic rejection | ||
| Mechanism of rejection | Natural Antibodies / Complement / Thrombosis | Antibody / Complement / Coagulation / Hemorrhage / | ||||
| Inflammation / T cell infiltrate / fibrosis | ||||||
| Innovation | None | Adsorption antibodies | Transgenes | Gene inactivation | Transgenes / gene inactivation | Tolerance ? |
| ( Complement proteins) | GalT | |||||
The natural immune barriers brought into play during xeno-transplantation
Pig-to-primate transplants (Figure 3), are subject to intense and rapid immunological rejection involving both innate and adaptive immune responses. Innate barriers involve monocytes and macrophages, Natural Killer (NK) cells, and complement and coagulation pathways [2].
Figure 3: Xeno-transplantations: Pigs and Non-Human Primates (PNH)
Furthermore, “natural” antibodies, which are present without previous exposure to known ligands (as are the natural antibodies of the ABO blood group system), constitute another formidable obstacle. The most dangerous natural antibodies recognize α-galactose-1,3-galactose (Gal). Gal corresponds to a carbohydrate sequence carried by many membrane glycoproteins and glycolipids of pig cells and most other mammalian species, with the exception of man and monkeys which do not carry such an epitope by following a du gene codantα−1,3-Galactosyltransferase (GalT) mutation in our ancestors. The high titers of natural antibodies against Gal in human serum probably reflect exposure (and immunization) to this sequence which also belongs, indeed, to many commensal microorganisms of the microbiome. In humans, anti-Gal antibodies represent 1 to 4% of circulating human immunoglobulins and include both IgM and IgG [3]. These natural anti-Gal antibodies were the major early obstacle to the first xeno-transplants. Anti-Gal antibodies are responsible for Hyper Acute Rejection (HAR), which occurs within minutes or hours following the completion of vascular anastomoses between the transplanted organ and the recipient [4,5].
B and T lymphocytes are the adaptive immune barriers in xeno-transplantation in primates
Tc cells (CD8+) can directly attack the graft and Th cells can also promote B and NK cell responses [6]. Direct activation of human CD8 + and CD4+ T cells by porcine MHC class I and class II molecules, respectively, has been described and is similar in magnitude to direct allow-responses seen in allografts.
Une reconnaissance indirect intervenient également dans la reaction immunities anti-porcine [7]. Cellules Présentatrices Antigènes (CPA) du receveur (primate) traitent et présentent les xéno-antigènes (peptides antigéniques) du donneur associés à des molecules du CMH du receveur. Cette reconnaissance indirect est plus forte que la response indirect aux allo-antigènes, conformément à la prediction d'un plus grand polymorphism protéique, et donc peptidique, entre espèces qu'au sein d'une même espèce.
Indirect activation of IFN-γ-producing recipient T cells is implicated in some rejection of porcine xenografts ein monkeys. The induction of specific antibody responses à against transplanted organs is largely due to the presentation of donor antigens by B cells to helper (Th) T cells in the recipient (cellular cooperation) [8].
Choosing the pig as an organ donor animal
As indicated in the introduction, the pig is now considered the most suitable xenograft donor animal, due to its size, its favorable reproductive characteristics and the similarity of several of its organs to those of humans [9]. We will consider the main characteristics of donor animals that are likely to be necessary for successful xenotransplantation.
Availablity
Unlike the non-human primates or PNH (chimpanzees, baboons) used as donors for the first clinical trials of xeno-transplantation, pigs are not an endangered species. More than 100 million pigs are slaughtered for food each year in the United States alone. Although there are animal rights groups that oppose the use of animals for any purpose, it seems unlikely that society would want to limit the number of pigs whose organs could be used for save the lives of human beings. However, not all pigs would be suitable for this purpose, as special breeding and care will be required to meet scientific and regulatory criteria [10].
Security
As in the case of allogeneic transplantation, the two most important safety issues for xenotransplantation are the risk of failure due to rejection and the risk of side effects due to the immunosuppressive drugs needed to prevent rejection, especially the risk of infection. At the end of the 1990s, discussions around xenotransplantation focused on the risk of transmission of infections from pigs to humans with the possibility that an infectious microorganism could be transferred to the recipient but also to relatives or contacts of the recipient, for example family members, medical or nursing staff. This could pose a potential risk to the community, especially since human beings may not have natural immunity against this microorganism.
These discussions largely resulted from the observation of a porcine kidney cell line capable of transmitting endogenous Porcine c-type Retroviruses (PERV) to human cell lines in vitro. It was feared that these porcine PERVs could, theoretically, infect human xenograft recipients and cause disease as a result of insertion of the retroviruses into the host genome, leading to disruption of normal gene function or oncogenesis [11]. Extensive research on the risk of human infection by PERVs has followed these observations. This research has shown that, in reality, primary human cells are very difficult to infect due to the presence of human restriction factors that limit the risk of PERV transmission. No PERV infections have been detected in more than 200 patients who have been exposed to porcine cells or xenografts over the past decades. Hundreds of non-human primates receiving porcine xenografts also showed no signs of PERV infection. In xenotransplantation the theoretical risk of infection with PERV can also be managed with appropriate oversight by national health regulatory agencies. However, it is possible to prevent the activation of PERVs by genetic engineering of the pig if this proves necessary (The activation of PERVs can be suppressed by small interfering RNA technology).
It can now be stated that porcine xenografts, when performed appropriately, carry less risk of infection than human allografts. In fact, deceased allograft donors are screened for a large number of known human viruses, but the urgency of using organs for an allograft from brain-dead human donors often makes it difficult to achieve sufficient security testing. Currently, approximately 0.2% of transplant recipients suffer from unexpected infections transmitted by the allograft. In contrast, rearing donor pigs in a carefully controlled and monitored environment can virtually eliminate this problem. The World Health Organization (WHO) and the International Eno I- transplantation Association (XIXA S) have developed guidelines and recommendations for clinical trials of xeno-transplantation. Recommendations include the use of a 'designated pathogen-free' pig that should be housed in a closed facility where the risk of introducing new pathogens is minimized: a bio secure facility known as a 'super clean'.
As a result of the experience acquired in immunocompromised subjects undergoing allo-transplantation of organs, when transplanting sorgans from pigs to non-human primates, prophylaxis (under the action of ganciclovir and Val ganciclovir) has also been considered to prevent activation of porcine cytomegalovirus (CMV) in recipients. However, donor pigs (particularly those from Revivicor's laboratory, Blacksburg, VA) have been bred to be CMV-negative which may preclude CMV prophylaxis.
Cut
One of the attractive characteristics of pigs as xenograft donors is that their organ sizes are matched to the size of any potential human recipient. However, the choice of the pig line that will be the most appropriate still remains to be à defined. Domestic pigs, which are used by most labs raising genetically modified pigs as potential xenograft donors, reach adult weights of over 450 kg so their organs would be too large (which, for example in the case of the kidney, conduirait cortical necrosis), for use in humans after about one year of age. In contrast, minipigs, which have been extensively developed for this purpose in several laboratories, attain maximum adult weights of 100-150 kg, approaching those of humans, making their organs potentially usable at any age.
Breeding characteristics
In addition to allowing large numbers of animals to be raised quickly and inexpensively, these breeding characteristics make du pigs one of the few large animal species in which it is possible to carry out a selective genetic breeding program. The truies have large litters (5 to 10 newborns), early sexual maturity (5 months), a short gestation period (114 days) and frequent cycles of estrus (every three weeks). Thus, it was possible to produce miniature transgenic pigs homozygous for MHC in a relatively short time. A genetically modified inbred line which has reached an inbreeding coefficient greater than 94% has also been created, allowing transplants from all the animals of this line.
Structural and physiological similarity with humans
The pig has been widely recognized as the optimal donor animal for human transplantation, largely due to its physiological similarities to the human species. Most pig tissues and organs bear a remarkable resemblance to those of humans, both in structure and physiology. This involves the heart and circulatory system (Figure 4), kidneys, pancreas, liver, lungs and even skin, qui is virtually indistinguishable histologically from human skin. Probably the main difference that needs to be considered in choosing the optimal donor animal is size, as outlined above. This factor may be more important for thoracic organs, which are confined in a closed cavity, than for abdominal organs, for which additional room for growth is seraitpossible. Nevertheless, excessive growth could lead to organs that would require increased blood supply, causing cortical necrosis already observed in the case of pig kidneys transplanted to animals of very different sizes.
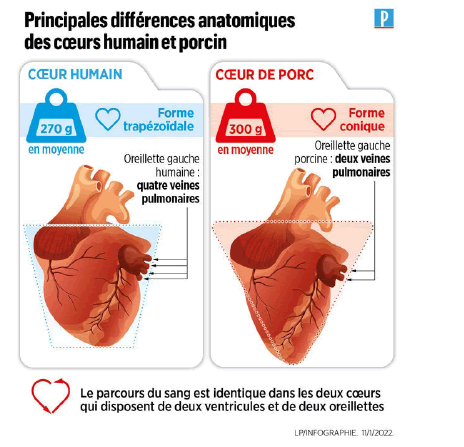
Figure 4: Anatomical differences of human and porcine hearts
However, organ-specific physiological differences are to be expected, particularly if the organ produces a species-specific molecule, such leas kidney-produced erythropoietin par or liver-produced clotting factors. As long as the number of these differences is limited for each organ, genetic modifications can overcome the incompatibility. As the survival of porcine organ xenografts is prolonged in non-human primates, however, previously unknown physiological incompatibilities may emerge and require appropriate treatment or additional porcine genetic modification.
Use of genetic engineering to overcome immune barriers to xenotransplantation
Given the large evolutionary distance between pigs and primates (estimated at 70 to 80 million years), it is amazing how similar these two species have remained, both physiologically and immunologically. Nevertheless, genetic mismatches that have arisen during evolution have resulted in incompatibilities that must be overcome for xenotransplantation to be successful. Fortunately, largely due to their favorable breeding characteristics, pigs are particularly susceptible to genome engineering. Enormous progress has been made over the past two decades in overcoming interspecies incompatibilities through genetic engineering. The genetic changes that have been introduced into pigs are summarized (Table 2 and Figure 5).
Table 2. The evolution of genetic engineering techniques applied to pigs used in xenotransplantation.
| Year | Technical |
|---|---|
| 1992 | Microinjection of randomly integrating transgenes |
| 2000 | Somatic Cell Nuclear Transfer (SCNT) |
| 2002 | Homologous recombination |
| 2011 | Zinc finger nucleases (ZFNs) |
| 2013 | Transcription activator-like effector nucleases (TALENs) |
| 2014 | CRISPR/Cas9 |
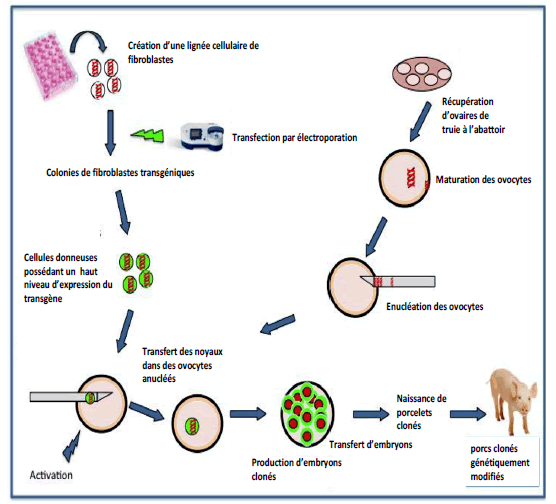
Figure 5: Cloning of genetically modified pigs
Two major issues must be taken into account, namely:
• Which genetic modifications (currently available) are optimal to reduce the risk of transplant rejection?
• And what measures should be taken to minimize the risk of transfer of an infectious microorganism from the graft to the immunocompromised recipient?
Genetic engineering techniques applied to pigs have evolved considerably over the past 3 decades. A wide range of genetically modified pigs have been supplied or offered as organ donors for experimental studies, which sometimes makes the results difficult to compare (even within the same study). One must identify the genetically modified organ donor pig that would be optimal (given our current knowledge) and transplant only the organs from these specific pigs. The exact pig phenotype must be confirmed in vitro before conducting an in vivo study.
From the beginning of the 2000s, the demonstration of the possibility of cloning by nuclear transfer in mammals (following the " Dolly " experiment), several laboratories have, for the first time, deactivated in fetal fibroblasts a porcine gene involved in the phenomenon of rejection: The GalT gene, then, from a culture of these genetically modified fibroblasts, carried out a nuclear transfer in enucleated ovules before implanting the resulting modified embryos in receptive sows. Porcine organs were no longer affected by high titers of anti-Gal in recipient primates, limiting the problem of hyper acute rejection of porcine grafts which significantly prolonged xenograft survival.
New advances in genome editing techniques: the use of ZNFs, TALENs and, more recently, CRISPR/Cas9 nucleases, have facilitated the modification of the porcine genome in such a way as to optimize the success of xenotransplantation. All of these techniques have been used to modify the genome by nuclear transfer from genetically modified somatic cells (Figures 6).
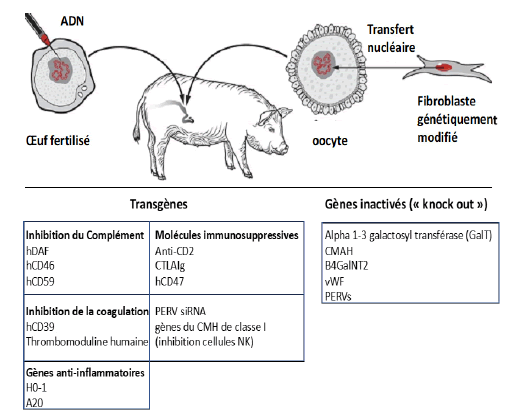
Figure 6: Genetic modifications made to pigs to facilitate transplantation of porcine organs to humans
The genetic modifications made to pigs are of two types:
• Inactivation or deletion of certain porcine genes which prevents their expression in the form of proteins which are involved in rejection reactions
• Insertion into the porcine genome of human genes which lead to the expression by the graft of human proteins, which also limits the risk of rejection.
Triple Knock -Out (TKO) transgenic pigs
Currently, most researchers consider that the suppression of the expression of genes encoding certain carbohydrate xenoantigens expressed in pigs and against which humans possess natural (preformed) antibodies is necessary. This is primarily the gene encoding α−1,3- Galactosyl Transferase (GalT) (Figure 7) which synthesizes the sequence Gal or α-gal (α-galactose-1,3-galactose). Anti-Gal antibodies are responsible for Hyperacute Rejection (HAR), which occurs within minutes or hours following the completion of vascular anastomoses between the transplanted organ and the recipient. Attempts at column adsorption or neutralization of these antibodies have made it possible to limit this rejection, leading to delayed rejection, but without long-term success. The elimination of the GalT gene in transgenic pigs called " Gal Safe " by nuclear transfer technology allowed the disappearance of HAR in nonhuman primates as early as the early 2000s. When HAR was successfully eliminated, by deletion of the GalT sequence from pigs using nuclear transfer technology, it was found that antibodies against porcine epitopes other than Gal could cause delayed vascular xenograft rejection, termed "acute vascular rejection" or "Delayed Xenograft Rejection (DXR)". This rejection occurs in the days or weeks following the transplant. The responsible natural antibodies, different from anti-Gal antibodies, can bind to several membrane antigens. Many of these antibodies recognize, in particular, two characteristic antigens: either the terminal carbohydrate Sda (similar to certain blood group antigens s) produced by a β-1,4 N-acetylgalactosaminyl transferase 2 (β 4GalNT2) or N-glycolylneuraminic acid (Neu5Gc), an epitope expressed by glycoproteins and gangliosides [3,4]. Neu5Gc is highly expressed on endothelial cells of all mammals except humans and natural anti-NeuGc antibodies are present in many human sera reflecting a point mutation in the human gene for cytosine Monophosphate-N-acetylneuraminic acid hydroxylase or CMP-NeuAc hydroxylase (CMAH) which produces the NeuGc epitope in other mammals.
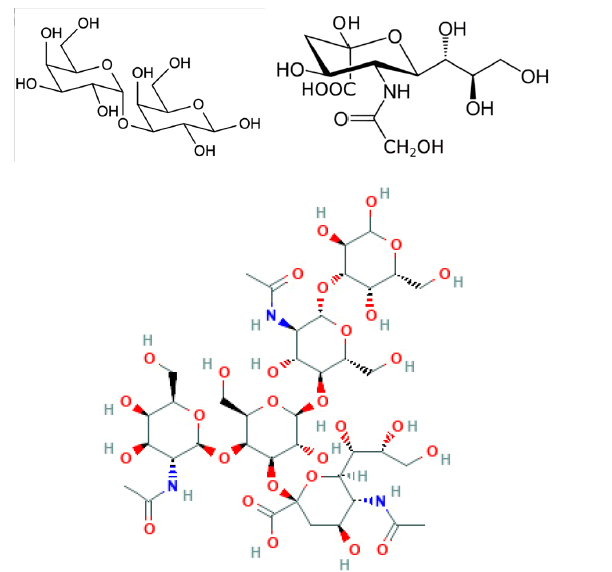
Figure 7: Galactose - -1,3-galactose, N-glycolylneuraminic acid and Sda antigen
The simultaneous deletion of the three genes encoding the Gal, Sda and NeuGC antigens respectively led to the creation of transgenic pigss triple knockout (TKO) who will, a priori, be, used as donors for all the first clinical trials in humans [4]. The natural antibodies of transplanted adult humans will no longer react against these three antigens, preventing hyperacute transplant rejection and limiting the risk of delayed transplant rejection (Table 3).
Table 3. Carbohydrate xenoantigens that can be eliminated or inactivated in transgenic pigs.
| Carbohydrate antigen | Enzyme | Knock out ” transgenic pig |
|---|---|---|
| α−1,3-galactose (Gal) | α−1,3-galactosyltransferase (GalT) | GTKO |
| N-glycolylneuraminic acid | Cytosine monophosphate-N-acetylneuraminic acid hydroxylase (CMP-NeuAc hydroxylase or CMAH) | CMAH-KO |
| (Neu5Gc) | ||
| nda | β-1,4 N-acetylgalactosaminyl transferase (B4GalNT2) | β 4GalNT2-KO |
Insertion into the porcine genome of human complement regulator transgenes
Although some researchers believe that Triple Knockout (TKO) pig organs alone may be sufficient to carry out clinical trials in humans, many others consider that the graft may still present other risks of injury, in particular as a result of the cytotoxic action of human complement activated after binding of antibodies to other porcine xenoantigens, leading to ischemia.
In humans, when the complement system cascade is activated, for example in response to the presence of pathogenic bacteria in the blood, complement activation does not usually damage host tissues. This is due to the expression of human Complement Regulatory Proteins (CRP) on the surface of vascular endothelial cells. Pigs have similar CRPs, but these are relatively ineffective in protecting pig cells from human complementmediated injury.
It would therefore seem judicious that the porcine graft could, by introduction into the porcine genome of transgenes of human origin, express one or more regulatory proteins of the human complement CRP, for example the proteins CD46 CD55 (or hDAF) and CD59, which can protect a porcine graft of the cytotoxic effects of human complement [5]. Under usual conditions, CRPs are expressed on human endothelial cells to prevent collateral damage that can affect host cells and modulate the inflammatory reaction when complement is activated during the destruction of microorganisms. To enhance these protective mechanisms in pigs whose organs would be exposed to human antibodies and complement, DNA constructs for three human CRPs have already been introduced into fertilized pig eggs by nuclear transfer. The expression of human CRPs by the endothelial cells of the transplanted porcine organ has made it possible, in non-human primates, to prolong the survival of the graft for several weeks after prior elimination of the natural antibodies by adsorption. Unfortunately, natural antibodies reappeared in the recipient, often in even higher titers, causing Delayed Xenograft Rejection (DXR).
Insertion into the porcine genome of human transgenes encoding antiinflammatory and/or anticoagulant proteins
The systemic inflammatory reactions often observed in the grafts are due to the action of various cytokines such as IL6 or TNF. This suggests that the transgenic expression of one or more human anti-inflammatory (anti-apoptotic) proteins could also limit the risk of rejection. For example, a transgene encoding human hemoxygenase-1 (hHO-1) has been added to the genome of donor pigs. This enzyme which converts heme into bilirubin, carbon monoxide and free iron promotes the protection of cells against oxidative damage through its anti-inflammatory, anti-apoptotic and antiproliferative effects through mechanisms which probably involve, at least in part, the production of CO. Survival of xenografts in NHPs was prolonged but not systematically. Transgenic expression of hA20 was also tested; it is a human TNF-induced protein enzyme that inhibits NF-kappa B activation and TNF-mediated apoptosis. Expression of this transgene in pigs has been restricted to the heart, where it appears to partially inhibit ischemiaperfusion injury, but without a significant increase in transplanted organ survival [11, 12].
The attack of vascular endothelial cells by natural antibodies (whose antigenic targets are still poorly understood), the deposition of complement and the attack of the endothelium by innate immune cells (neutrophils, monocytes, macrophages) lead to the development of a thrombotic microangiopathy that contributes to graft rejection due to fibrin deposition and platelet aggregation in the graft vessels. Consumption of the recipient's clotting factors when they are depleted can result in "consuming" coagulopathy leading to spontaneous bleeding (eg, into the gastrointestinal tract) which can be fatal. Nevertheless, intense immunosuppressive therapy, reducing the immune response at the level of porcine vascular endothelial cells, may also delay the development of thrombotic microangiopathy. This supports the conclusion that the development of this complication is mainly related to the activation immune system and endothelial damage.
Incompatibilitiesdescribed in coagulation systems between pigs and primates also suggest that transgenic expression of at least one human coagulation regulatory protein, e.g., Human Thrombomodulin (hTBM) (CD41), the endothelial protein C receptor (EPCR) or the Tissue Factor Pathway Inhibitor (TFPI) would limit the risk of rejection in transplant patients [13-15]. The expression of these proteins could prevent the development of a thrombotic microangiopathy within the transplanted organ or of a coagulopathy in the recipient. The transgenic expression of human thrombomodulin expressed on the surface of endothelial cells has already contributed to the long-term survival of cardiac xenografts in recipient baboons.
Moreover, the suppression (knock out) of the expression of the porcine von Willebrand factor (vWF) could also protect the grafts. vWF is a glycoprotein that induces platelet adhesion to sites of vascular injury by binding to and stabilizing coagulation factor VIII. This suppression of the vWF factor could limit the risk of coagulation at the interface between the blood of the transplanted patient and the porcine endothelial cells. This protective function has been demonstrated ex vivo but its effect on xenograft survival has not yet been established.
Finally, the transgenic expression of the enzyme hCD39 (human ectonucleoside triphosphate diphosphohydrolase-1; ENTPD-1), which hydrolyses ATP and ADP to AMP, which is itself hydrolyzed to adenosine, was considered. hCD39 has been shown to have anti-thrombotic and cardiovascular protective effects. The hCD39 transgene has been included in several pig-primate xenograft models.
Prevention of T cell-mediated rejection
Regardless of the genetically modified pig used as the organ donor, in the absence of immunosuppressive therapy, the recipient will develop a T cell-dependent (adaptive) immune response that leads to infiltration of the graft with T cells and other immune cells as well as d’anti-pig antibody secretion (essentially IgG). This response will inevitably lead to transplant rejection. Consequently, an effective immunosuppressive diet must necessarily be administered to the recipient subject to prevent this response, which does not dispense with genetic modifications applied to the donor pig to slow down the specific immune response.
Surprisingly, genetic modifications applied to donor pigs chosen to dampen the recipients' innate immune response (PNH) also, in some cases, reduce the adaptive immune response. Thus, deletion of Gal antigens or expression of a human CRP in transgenic pigs significantly reduces the in vitro response of T cells to porcine cells, but this is difficult to confirm in vivo.
The use of genetic engineering to dampen recipient T-cell immune responses and even render xenogeneic organs immune to immune attack is attractive. The approaches that have been explored include the introduction into the donor pig of transgenes encoding FasL (Human FAS ligand) (CD95L), CTLA4Ig (CD152) and anti-CD2 monoclonal antibodies, with the aim of obtaining immunosuppression locally after transplantation [16,17].
CTLA4-Ig, in particular, is a fusion molecule associating the Fc fragment of a human IgG1 with a T lymphocyte membrane antigen: CTLA4 T Cytotoxic T-Lymphocyte Antigen 4. This molecule prevents the interaction between APCs and T cells, inhibiting recipient T cell activation (Figure 8). The transgene has been shown to prolong graft survival in non-human primates. However, evasion of immune responses sat the xenograft level may impair immune protection against infections or viral reactivations in genetically modified pigs. These mutagenic constructs can thus affect the health of transgenic pigs. To limit this risk, methods to express the transgene only in specific cell types have been developed in pigs that express, for example, CTLA4-Ig only in vascular endothelial cells.
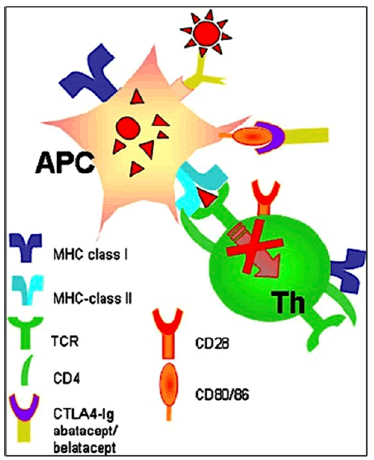
Figure 8: The CTLA4-Ig molecule prevents the interaction between APCs and T cells, inhibiting recipient T cell activation.
Another approach to reduce T cell involvement and activation is to inactivate or reduce the expression of porcine MHC molecules (ALS antigens) class I and/or II, which would likely reduce the intensity of the adaptive immune response, but may be associated with immunodeficiency in pigs with increased susceptibility to infectious complications, for example, herpes viruses, in the pig graft itself. The deletion of class I MHC molecules has already been carried out in a transgenic pig line (MHC-1-KO).
Overexpression of human CD46 and CD47 antigens sin transgenic pigs, with potential for "immune masking" that prevents macrophage activation and phagocytosis of transgenic pig cells, was also tested. Tri-gene (GTKO/ CD46/CD47) transgenic pigs have been generated that express both CD46 and CD47 for use in PNH models.
Other examples of the use of genetic engineering in donor pigs
There are several other areas where pig genetic engineering can be useful. Some of them are of particular interest.
As the potential risks of the presence of Porcine Endogenous Retroviruses (PERV) in pigs remain unknown (although probably low), the inactivation of these viruses from PERV expression would be an advantage. Such genetic manipulation would eliminate the long-standing concern that PERVs could be pathogenic in humans or could combine with human endogenous retrovirus fragments to form new viruses. Researchers have thus combined the PERV - knockout with the genetic manipulations necessary to curb the innate immune response.
Following pig kidney (or heart) transplantation in PNHs (baboons), rapid growth of the organ was observed during the first few months, suggesting that innate factors, e.g. persistence of the porcine growth hormone, continue to function in the graft during this period of time. After about 3 months, however, this rapid growth diminishes and the growth of the organ becomes comparable to that of baboon-specific organs. UA knockout of the porcine gene encoding the growth hormone receptor may, however, be included in order to prevent the proliferation of the donor organ.
Another example concerns HLA-E molecules which have an immunosuppressive power. In vitro, the insertion of a transgene encoding a human HLA-E molecule protects endothelial cells from lysis by human NK (Natural killer) cells. In the context of xenogenic transplants, endothelial cells from genetically modified pig grafts could become insensitive to the lytic action of NK cells involved in rejection.
Macrophages also contribute to the rejection reaction. Introduction of human CD47 into transgenic pigs may reduce phagocytosis of xenogeneic cells (particularly hématopoïétiquesgraft-supplied porcine cells) by human macrophages [12].
Immunosuppressive, anti-inflammatory and anti-infective treatments for the transplanted patient
As in the case of allografts, immunosuppressive and anti-inflammatory treatments are necessary in the subject receiving a xenograft [18].
Adaptive immune response
The adaptive immune response of the recipient (male or PNH) dependent on T lymphocytes will lead to infiltration of the graft with T and B lymphocytes leading to graft rejection. To prevent this phenomenon, in humans, in the days preceding transplantation, during clinical trials, a depletion of T lymphocytes is planned by administration of an antilymphocyte serum or thymoglobulin (ATG: anti-thymocyte globulin) to lower the recipient's CD3+ T lymphocyte count less than 500 cells/ mm 3. Similarly, anti-CD20 monoclonal antibodies (rituximab) will be administered to reduce the level of B lymphocytes before transplantation.
Mycophenolic acid (mycophenolate mofetil) is another selective immunosuppressant tested in NHPs that could be used in clinical trials: it is a non-competitive and reversible inhibitor of inosine monophosphate dehydrogenase; it inhibits, without being incorporated into DNA, the de novo synthesis of the nucleotide guanine (Figure 9). Since the proliferation of B and T lymphocytes is essentially dependent on the synthesis de novo purines, and that other types of cells can use "replacement" metabolic pathways, mycophenolic acid has a more marked cytostatic effect on lymphocytes than on other cells.
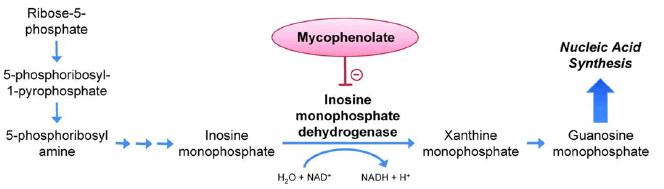
Figure 9: Mechanism of action of iscalimab (anti-CD40 monoclonal antibody)
After transplantation an anti-CD40 monoclonal antibody (Iscalimab) will be necessary during the first weeks following the transplantation; By binding to the CD40 receptor carried by Antigen-Presenting Cells (APC), which are responsible for activating B and T lymphocytes, this antibody will limit the risk of an immune reaction against graft antigens.L’iscalimab, anticorps dont la portion Fc est inactivée empêche l’interaction entre le CD154 des lymphocytes T et le CD40 des CPA et des macrophages (Figure 10).
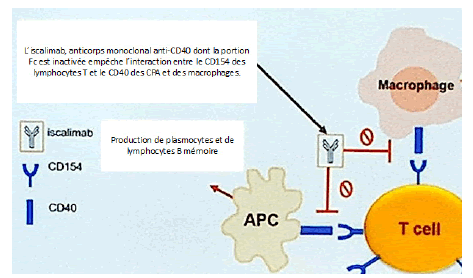
Figure 10: Mechanism of action of iscalimab (anti-CD40 monoclonal antibody)
Inflammatory response and infectious complications
Organ transplants from pigs to non-human primates are often followed by a systemic inflammatory response. Anti-inflammatories such as TNF (pro-inflammatory cytokine) inhibitors, eg etanercept, and IL-6 inhibitors, eg tocilizumab, which prevents the binding of IL-6 (another pro-inflammatory cytokine) to NHP tissues but not to pig tissues, could be added to the treatment. But the results are not yet convincing. Methylprednisolone (corticoid) is also considered in men.
Adjunctive treatments are also planned (aspirin, low molecular weight heparin, etc.)
Faced with the potential risk of infectious complications, prophylaxis (under the action of ganciclovir and Val ganciclovir) will undoubtedly have to be carried out to prevent the activation of porcine Cytomegalovirus (CMV) in the recipient (this is already done in grafted PNH).
In baboon’s progress has been made in cardiac xeno-transplantation, with a survival of more than 6 months of grafts from Triple Knock-Out (TKO) transgenic pigs, expressing human CD46 and human thrombomodulin.
These NHPs were subjected to a complex immunosuppressive treatment with rituximab, anti-lymphocyte serum, anti-CD40 antibodies, mycophenolate mofetil and corticosteroids. Myocardial enlargement was an important initial limitation to long-term survival, but this was limited by maintaining low blood pressure in the recipient by administration of an additional immunosuppressant, rapamycin; known for several decades, rapamycin is extracted from seaweed harvested on Easter Island. It is an effective immunosuppressant.
Tolerance
Advances in genetic engineering and immunosuppressive treatments have made it possible to prolong the maintenance of xenografts in nonhuman primates, but rejection-free graft survival is still unpredictable in the long term despite the use of high doses of immunosuppressants. The induction of tolerance, defined here as the absence of a destructive response to the graft in the absence of global immunosuppression, has become a new goal that would guarantee the long-term success of xenotransplantation. Two approaches to inducing this tolerance have shown promise in non-human primates: mixed hematopoietic chimerism and porcine thymic transplantation.
Mixed hematopoietic chimerism
Mixed chimerism refers to a state in which hematopoietic cells from the donor and the recipient coexist. It can be achieved by transferring hematopoietic cells from the donor (e.g. from bone marrow) to a recipient who receives immunosuppressive therapy that does not eliminate the host's hematopoiesis but is sufficient to make " place” for the donor marrow transplant. Mixed chimerism is associated with the induction of tolerance to organ transplants from the donor (Figure 11).
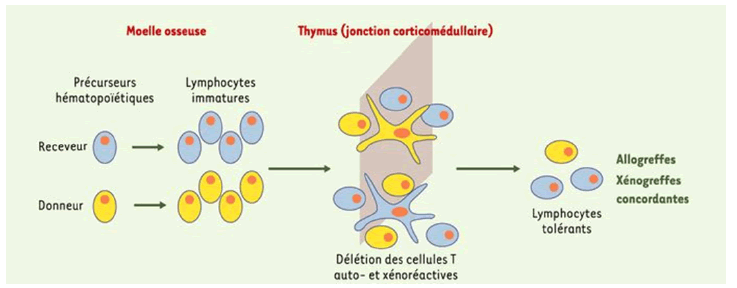
Figure 11: Donor bone marrow transplantation prior to organ transplantation can induce hematopoietic chimerism allowing deletion of xenoreactive T cells
Once the xenogeneic hematopoietic cell transplantation has been performed, the pre-existing natural antibodies (IgM) against the donor disappear in the mixed xenogeneic chimeras. This is a specific induction of B cell tolerance (energy). In baboons, mixed chimerism prevented all types of vascularized xenograft rejection: hyper acute, acute or chronic vascular rejection [19,20]. Thus, mixed chimerism has the ability to render recipient reactive T cells graft-tolerant, also preventing induced antibody responses, but also the action of natural antibody-producing B cells independently of T cells.
It should be noted that once xenogenic chimerism has been reached, the NK cells involved in rejection reactions also demonstrate donor tolerance.
Thymic transplant
An alternative to mixed chimerism for inducing T cell tolerance is transplantation of the donor thymus at the same time as the transplant. In the thymus, potentially self-reactive T cells, through a process of negative selection, are suppressed or inactivated (energy) after exposure to selfantigens (self-antigens) presented to them: this process plays a key role in self-tolerance.
Graft antigen tolerance can similarly be ensured by concomitant thymus grafting. For example, a graft of thymic tissue from a fetal pig transplanted into baboons thus resulted in donor-specific hyporesponsiveness and prolonged survival of the porcine skin graft associated with a depletion of T lymphocytes directed against the donor. Thymic transplantation can be coupled with kidney transplantation (Figures 12 and 13): “Thimokidney” (TK) Experiments using this method have been carried out with TKO porcine donors. The improvement in outcome was remarkable, with renal xenograft survival increasing to over 80 days and most animals dying for reasons other than rejection [20,21].
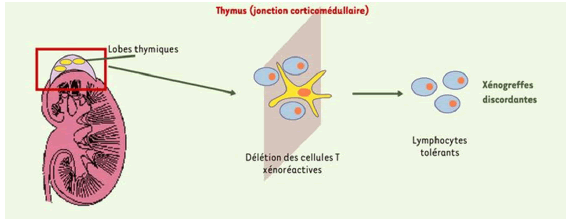
Figure 12: A transplant of fragments of porcine thymus from the donor under the renal capsule of the recipient before organ transplantation allows the re-creation of a vascularized porcine thymus which operates a deletion of autoreactive T lymphocytes.
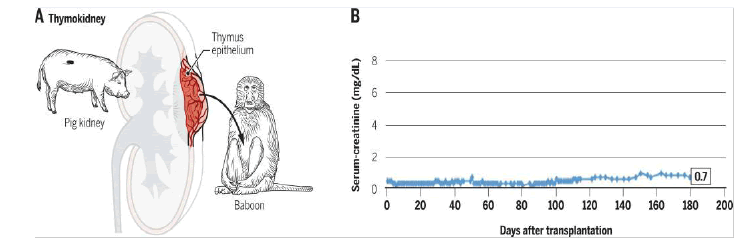
Figure 13: A. Diagram of thymokidney transplant from « thymus – rein » ou pig to baboon. B. Long-term survival of graft from TKO minipig in baboon with waning immunosuppression. Serum creatinine témoin de la fonction rénale was normal for more than 6 months and the graft developed well; it was destroyed by cortical necrosis without signs of rejection on day 193 after transplantation.
The causes of the death of David Bennett, transplanted with a pig's heart
Last March, two months after the transplant at the University of Maryland Medical Center, no obvious cause had been identified to explain the death of David Bennett. But on April 20, we learned that the transplanted heart was infected with a porcine cytomegalovirus, which would have had devastating effects on the transplant, leading to irreversible cardiac damage and the death of the patient.
Cardiac lesions caused by porcine CMV had already been observed in pig hearts transplanted into baboons which survived only a few days as a result of the infection. The rapid proliferation of the virus has led to irreversible dysfunction of the transplanted hearts. In the case of David Bennett, as in the baboons, the immunosuppressive treatments used to limit the risk of rejection probably contributed to the importance of the infection.
However, the transgenic pigs used for xenotransplantations are supposed to be free of porcine viruses (CMV, PERV in particular). The transfer of porcine viruses to humans remains, in fact, a source of concern: xenotransplantation could even, according to researchers, trigger a pandemic if a porcine virus adapts inside the body of the recipient, then spread to doctors and nurses. This risk may require lifelong monitoring of transplant patients. The presence of CMV in the transplanted heart is believed to be due to a failure of the biotech company, Revivicor, which bred and engineered the transgenic pigs used for xenotransplants. Revivicor declined, at this time, to comment on the failed transplant.
Virological tests were carried out regularly on the donor pig before transplantation and then on biopsies of the transplanted organ.
Did these tests lack sensitivity? CMV is, in fact, a latent virus that is difficult to detect. The American team that carried out the transplant seems to have looked for the virus only on a sample from the pig's snout but not from the internal organs which probably harbored the CMV. The medical team constantly monitored the patient's condition with a range of powerful blood tests. A DNA sequencer made it possible, in particular, to “scan” the patient's blood in search of soluble fragments of pig genes which would have signaled damage to heart cells. Another test, developed by the Karius company, made it possible to regularly analyze the patient's blood in search of traces of numerous infectious agents, in particular porcine viruses. It is this test, carried out on a blood sample taken from D. Bennett 20 days after the transplant, which gave an alert signal indicating the presence of cytomegalovirus pigs. But the signal strength was so weak that the medical team initially thought the result might be wrong (the pigs were supposed to be guaranteed germ-free, raised in bio-secure, so-called “super clean” facilities). Furthermore, the time required to obtain the results was 10 days after the sample was taken, which prevented the infection from being treated early enough. The heart failure resulted in a "cytokine cascade " as the infection spread throughout the heart. Treatment with a drug of last resort, cidofir (sometimes used for AIDS patients) and human immunoglobulins from donors could only prolong D. Bennett's survival for a few days. Doctors now fear they may have erred in administering human antibodies, these blood products containing natural anti-pig antibodies which may also have damaged the heart It is difficult to say that CMV is the sole cause of heart damage irreversible observed, the patient being very weakened before the operation. But the biopsies taken until death showed no signs of immune rejection, which would prove that the treatments of the donor pig and the patient were effective.
The detection of porcine virus in Bennett's heart is perhaps paradoxically good news for the future of xenotransplantation if it is the main cause of transplant failure. United Therapeutics companies and eGenesis plan to start clinical trials using pig organs within the next year or two.
The remarkable clinical success of transplantation over the past decades has led to a growing shortage of transplantable human organs, increasing the waiting list of patients aware that transplantation could save their lives. Although there are other competing technologies also in development, xeno-transplantation is probably the best short-term solution to this organ shortage. Many of the obstacles that previously impeded progress in xenotransplantation have now been overcome through genetic engineering applied to pigs to make their organs more compatible with the human immune system and physiology. Currently, it cannot yet be asserted that the use of clinically acceptable levels of immunosuppression could allow survivals of xenografts from transgenic pigs as long as those of allografts. The first two xeno-transplantations of porcine origin carried out in humans a few months ago will make it possible to better understand the optimal procedures:
The first intervention in humans, a kidney transplant in a clinically dead patient, was performed in September 2021 at the University of Alabama (USA); the transplant was maintained for three days, the kidney being transplanted under the thigh of the recipient: during this period, the production of urine from the pig kidney was in good conditions and the organ effectively filtered the blood of the patient, as a normal kidney would. No hyper acute rejection reaction was observed during the clinical trial, with the organ “flushing with the rush of blood and oxygen” as soon as circulation was established in the transplanted kidney.
The second intervention, a heart transplant from a transgenic pig was performed at the beginning of January 2022 at the University of Maryland (USA). The transplant was performed on a compassionate basis, as no alternative was possible for the patient who survived two months. During this period, no rejection reactions were observed and the causes of death are still uncertain. The patient was very fragile before the operation and the transplanted heart may not have been enough to keep him alive any longer. The transgenic GalSafe donor pig was, as in experiments carried out for many years in non-human primates, “Triple Knockout” (TKO) and possessed in its genome 6 human transgenes coding in particular for Complement Regulatory Proteins (CRP). A porcine gene (encoding a growth hormone receptor) had also been inactivated to limit the growth of the transplanted heart. It is likely that further genetic modifications will be made to the transgenic pigs that will be used for future human clinical trials. Immunosuppressive treatments will also probably be improved. The University of Maryland Hospital plans to continue its clinical trials.
[Google scholar] [Cross ref]
[Google scholar] [Cross ref]
Citation: Brunet JP. Xeno-Transplantation in Humans. Med Rep Case Stud. 2023, 08 (1),01-11.
Received: 09-Jan-2023, Manuscript No. MRCS-23-86174; Editor assigned: 10-Jan-2023, Pre QC No. MRCS- 23- 86174 (PQ); Reviewed: 19-Jan-2023, QC No. MRCS- 23-86174 (Q); Revised: 22-Jan-2023, Manuscript No. MRCS- 23-86174 (R); Published: 24-Jan-2023, DOI: 10.4172/2572 5130.23.8(1).1000233
Copyright: ©2023 Brunet, JP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.