Case Report - (2025) Volume 11, Issue 2
Extranodal Natural Killer/T-cell Lymphoma (ENKTCL)-associated Hemophagocytic Syndrome (HPS) is a rare and fatal disease. Chemotherapy was commonly used but the efficacy was dismal. Although immunotherapy with immune checkpoint inhibitors was effective in NK/T cell lymphoma, the use of immunotherapy in introduction treatment of HPS was controversial with concern on side effects. We report a case of HPS who was treated with immunotherapy instead of chemotherapy for HPS. The patient achieved complete remission, confirming that immunotherapy is an effective treatment approach for patients with ENKTCL-associated HPS.
Extranodal natural killer/T-cell lymphoma • Hemophagocytic syndrome • Immunotherapy
Hemophagocytic Syndrome (HPS), also known as Hemophagocytic Lymphohistiocytosis (HLH), is a rapidly progressive and highly lethal disease, with a median survival time of less than 2 months without treatment [1]. HLH clinically presents with fever, hepatosplenomegaly, suppressed blood cell counts, altered coagulation cascade and multi-organ dysfunction. HPS can be primary or secondary, which is induced by specific infectious or malignant cancer. Epstein-Barr Virus (EBV) is the most common infectious agent in patients with the viral-associated HPS.
ENKTCL-associated HPS is uncommon, life-threatening and is associated with a poor prognosis, furthermore, there is no specific treatment regimen. The current standard treatment of HPS was HLH 2004 protocol, which consists of a decrescendo course of etoposide and dexamethasone in the introduction therapy setting, followed by chemotherapy regimen specific for ENKTCL [2]. The outcomes vary in littering review, around 18% patients died within 2 months and the cause of death were reported to be related to associated lymphoma. Therefore, more effective strategy is needed.
PD-1 blockade reverses EBV cancer-related immunosuppression, thereby restoring the immunity required to restrain cancer cells and to clear EBV infection. However, there’s no study consider integration of PD-1 blockade in the induction therapy setting for ENKTCL-associated HPS.
We report a case of ENKTCL-associated HPS who was untoteralted to HLH2004 protocol, and responded well to PD-1 inhibitors in the introduction therapy setting.
A 61-year-old lady was admitted to local hospital due to fever for 1 month. She presented with "right lower leg skin ulceration for 2 years and fever for 1 month" on April 7, 2023. She had a history of Nasopharyngeal Carcinoma (NPC), undifferentiated non-keratinizing carcinoma, by nasopharyngeal biopsy on January 30, 2019. She received concurrent chemoradiation therapy at a cancer hospital in China mainland from February 13 to May 15, 2019. The efficacy was Complete Remission (CR). During follow-up she underwent serial PET/CT scan which revealed no signs of tumor relapse. Review of other systems was non-contributory. She denied any family history of malignancies. Two years before this admission, she developed right lower leg skin ulceration and she was treated with traditional Chinese medicine treatment but the skin lesions were consistently presented. Most recent PET/CT scan in August 2022, with the purpose for follow-up for NPC, revealed no recurrence of NPC. In November 2022, a skin lesion biopsy was performed at an external hospital, the pathological diagnosis found skin lesion was consistent with non-specific peripheral T-cell lymphoma. ISH study found tumor was positive for EBER. The tumor tissues were sent to second hospital for pathology consultation, the result was consistent with original findings (Figures 1 and 2).
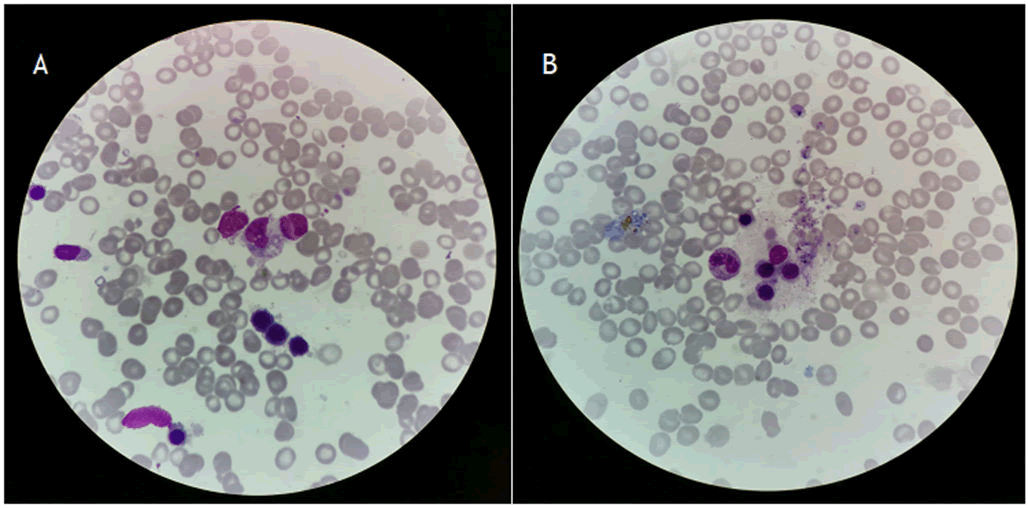
Figure 1. A and B represents the hemophagocytic cells in the bone marrow.
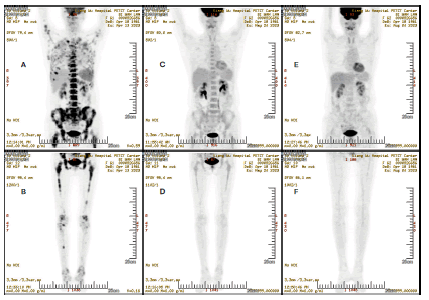
Figure 2. PET/CT scan image for efficacy evaluation. (A and B): Upper and lower of body before treatment; (C-F): Represent upper and low body from 1 month and 4 months after treatment, respectively
Immunohistochemical analyses demonstrated CD2 positive (weak), CD3 positive, granzyme B positive, MYC positive (weak), perorin positive, TIA-1 positive, EBER ISH positive, ki-67 approximately 90% cells positive, ALK negative, BCL2 negative, BCL6 most cells negative, CD4 negative, CD5 negative, CD7 negative, CD8 negative, CD10 negative, CD20 negative, CD30 negative, CD34 negative, CD56 negative, CD 68 negative, CD138 negative, CXCL 13 negative, cytokeratin negative, PAX5 negative, PD1 most cells negative, TCRgamma negative, TdT negative.
One month prior to admission, the patient developed recurrent fever, with a maximum temperature of 40°C and night sweats
Laboratory findings on admission included a White Blood Cell count (WBC) of 1.47 × 109/L, Neutrophils (Neu) of 76.9%, Hemoglobin (HGB) of 87 g/L and Platelets (PLT) of 114 × 109/L. Hypertriglyceridemia (triglyceride: 3.34 mmol/L); serum ferritin: 6712.00 ug/L; fibrinogen decreased: 1.32 g/L. EBV was negative with a low replication rate (<5 × 102 U/ml). Her basic metabolic panel, liver function tests, amylase and lipase levels were within normal limits.
PET/CT revealed nasal cavity mass with hyper metabolism, multiple foci of skin and subcutaneous lesions on the right lower leg, involving nasal cavity, subcutaneous lesion on both lower leg and extensive bone marrow involvement. A bone marrow aspirate revealed hem phagocytosis, fulfilling 6 of the 8 criteria for HLH-2004, leading to the diagnosis of Extranodal Natural Killer/T-Cell Lymphoma (ENKTCL) associated HPS according to criteria.
Treatment strategy
The patient was treated with dexamethasone 10 mg/day for 7 days and VP16 100 mg/M2 for 2 days. However, her CBC was not improved, and she presented with fever and significant fatigue. She was unable to tolerate further chemotherapy agent such as etoposide or P-GEMOX chemotherapy regimen. Therefore, immunotherapy with Pembrolizumab 100 mg was initiated as an alternative treatment. After the immunotherapy, the patient's blood count fully recovered, and she was treated with Gemzar 1.4 g monotherapy on May 2, 2023. Subsequently, she received Oxaliplatin 100 mg chemotherapy. After 4 courses of Pembrolizumab combined with GEMOX chemotherapy.
One month later, a PET/CT scan revealed Good Partial Remission (GPR) and further PET/CT scan confirmed CR. She received maintenance Pembrolizumab therapy for 1 year. A recent PET/CT found she keeps CR and in Disease Free Survival (DFS) status (Figure 3).
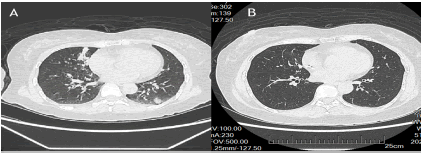
Figure 3. CT scan image of irAE of lung. (A): Showing nodular in bilateral lung; (B): Resolving of nodular.
Lymphoma-associated HLH is a highly fatal disease, with the mortality of about 90%. Previous study demonstrated that patients with ENKTCL-associated HPS had a 6-month Overall Survival (OS) rate of merely 34.4% [3]. The strategy to treat ENKTCL-associated HSP including introduction therapy and treatment for ENKTCL. An 8-week induction therapy including etoposide (VP-16) and dexamethasone are critical elements in treating HLH in the HLH-2004 guidelines. However not all patient responded to this protocol, due to rapid progression of ENKTCL [4]. Recent study has found that patients who responded to the ENKTCL treatment also achieved responses to accompanied HPS, thus prompt treatment targeting ENKTCL rather than HLH in patients with ENKTCL-associated HLH is recommended [5].
The central dilemma for EBV-HLH treatment is that chemotherapies that aim to eliminate the hyperactive immune cells responsible for the clinical phenotype also impair the ability of patients' immune systems to clear the underlying EBV infection which drives the disease. Immunotherapy with PD-1 inhibitors is highly effective in EBV-related lymphomas, make it an ideal treatment opinion in EBV-related HPS. Addition of anti PD-1 inhibitors to PGEMOX was found to be highly effective in advanced Extranodal Natural Killer/T-Cell Lymphoma (ENKTCL) with 66.7% of patients having durable CR [6].
Another recent retrospective study found nivolumab was safe and effective in relapsed or recurrent lymphoma-associated HPS, CR was seen in 5 of 7 patients, molecular test confirmed nivolumab restore the expression of HLH-related costimulatory and degranulation genes that were previously repressed in this cell population [7]. However, there were some case reporting PD-1 inhibitors induced HPS in treatment other cancers [8,9].
To our best knowledge, it’s first report to integrating anti PD-1 inhibitors for introduction therapy of newly diagnosed ENKTCL-associated HPS. In this case ICIs was safe and we tolerated. This provide an opinion for patients who cannot tolerate etoposide in introduction therapy of ENKTCL-associated HPS. More study is needed to explore biomarkers of sensitive patients.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Cao Y. "Use of Immunotherapy in Introduction Therapy of Hemophagocytic Syndrome Induced by Extranodal NK/T-Cell Lymphoma: Case Report". Oncol Cancer Case Rep, 2025, 11(1), 1-2.
Received: 09-May-2024, Manuscript No. OCCRS-24-134415; Editor assigned: 11-May-2024, Pre QC No. OCCRS-24-134415 (PQ); Reviewed: 25-May-2024, QC No. OCCRS-24-134415; Revised: 13-Mar-2025, Manuscript No. OCCRS-24-134415 (R); Published: 20-Mar-2025, DOI: 10.35248/2471-8556.25.11(1).001-010
Copyright: © 2025 Cao Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.