Mini Review - (2020) Volume 11, Issue 7
The effects of music on working memory among older adults: Literature review
Fereshteh Bagheri*
*Correspondence:
Fereshteh Bagheri,
Department of Audiology, School of Rehabilitation Sciences, Babol University of Medical Sciences, Mazandaran,
Iran,
Tel: 0000-0003-4419-4524,
Email:
Author info »
Abstract
Older adults demonstrate a decline in working memory which in turn leads to a reduction of cognitive skills. Therefore, perform optimal approach of working memory remediation is important to well-being for older adults. This paper aims to review the effects of music on working memory among older adults as well as the role of working memory in the central auditory system. Articles included in this review were identified through a search of the databases PubMed, Scopus, and Google Scholar using the search terms music, working memory, aging and central auditory processing disorder. The literature search was restricted to the years 1981 to 2020 and articles published in the English language. Central auditory processing skills such as speech-in-noise perception impaired mostly among older adults. Early diagnose of central auditory processing disorder and perform the music therapy is very important in older adults.
Keywords
Music • Working memory • Central Auditory Processing Disorder
• Aging
Introduction
The population of elderly people is increasing all over the world and also the
difficulty involving of mental health is rising in aging [1]. It is anticipated that
the population of older adults (above the age of 60) reach 2.1 billion in 2050.
Aging is associated with age-related changes in function of various parts of
the body such as cognitive system that lead to limited social activity, lonely
and physically weak [2,3], so our mission must be to study about effective
prevention and treatment protocol in order to create good living conditions
and increase of independence in old age [4-6].
One of the important complaints in aging population linked to loss of
memory and substantial part of difficulties in memory system attributed to
working memory (WM) that its function change with aging [7,8]. WM decline
is component of the normal aging process and it happens in healthy older
adults, so not related to presence or absence of neurologic disorder [9,10].
However those of elderly with WM decrease are at the risk of developing
neurodegenerative disorder such as mild cognitive impairment (MCI) and
Alzheimer disease [11,12]. Although the decline of WM in healthy older
adults has negative effects on life performance and can cause increase of
dependence but these effects are far greater on elderly with neurodegenerative
disease [13,14].
The aim of this study is to highlight the importance of the WM role in daily
activity among elderly and consider the optimal approach to reduce the
effects of WM capacity decline in aging.
Method
Of 288 primary articles, 103 potentially eligible articles were reviewed. Articles
included in this review were identified through searching the databases
of PubMed, Medline, Scopus, Google Scholar and Scientific Information
Database using the search terms of music, working memory, aging and central
auditory processing disorder. We considered the factors related to working
memory, such as aging. The literature search was restricted to the years 1981
to 2020 and the English language. Figure 1 shows the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of
study selection.
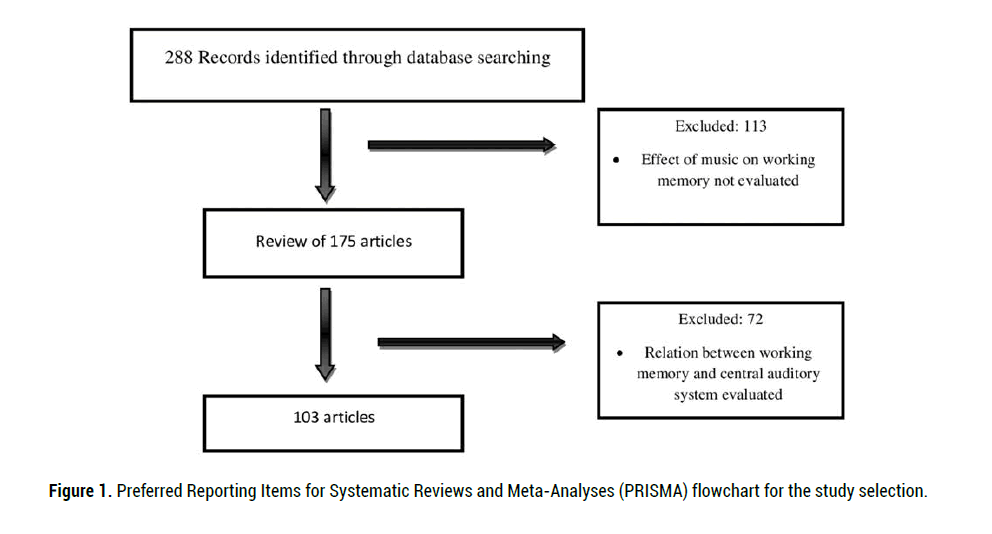
Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the study selection.
Results
Working memory and aging
Many parts of the brain proportionally activated for working memory execution
[15]. Various skills are necessary for the WM performance such as attention,
sort-term memory and utilization of verbal and auditory information. This
information maintained in WM for phonological representation of language so
WM is fundamental for convey of information (word, letter and number.) [16].
Among the skills that required for WM performance, attention has great importance
and age-related WM decline has been related to impairment of selective attention
[17]. By definition, selective attention is the process to concentrate on target
message while ignoring untargeted message. Selective attention guides our
awareness toward the task and keeping the information in WM [18].
Because of the vital role of WM in higher level cognitive processes the
capacity of WM is very important for higher level cognitive considers [19,20].
Higher-level cognitive functions related to various features of everyday
action. Therefore, WM impairment can lead to decline of every day functioning
[21,22]. WM is one of the core executive functions that involved in planning
[23,24]. WM involved in complicated cognitive functions such as learning and
language perception [25]. WM includes of verbal, visuospatial and executive
function that verbal and visuospatial categories are more susceptible to
aging than the other executive system [26-28] and visuospatial is most agesensitive
[29,30], of these components executive system is more related to
prefrontal cortex [31,32].
Several studies have shown that WM linear decreases with age [33-37] and
this decline essentially related to impairment of processing ability [38]. In
other word WM decrease along with other cognitive mechanisms such as
processing speed in aging [39-41]. Information manipulation is more agesensitive
than information maintenance [42].
Older adults with limited WM capacity exposed to great volume of linguistic
materials, this situation probably lead to impairment of cognitive process and
difficulty with perception of complex sentences [43,44].
WM is necessary for everyday tasks such as take medication so the role of WM
is very important in older adults. Thus, those of older adults with condition
that required taking medication exposed to greater risk for overlooking to take
medication [45].
Age-related changes begin in some components of the brain that played role
during WM performance [46]. These changes affect function and structure of
the brain, however the exact mechanisms of these changes are unknown, but
gradually decline of neural cell begins in prefrontal (in particular, dorsolateral
prefrontal cortex) and parietal regions [47-51]. Each one of these regions
has particular role in WM, the frontal regions at the monitoring situation, the
parietal regions at the utilization of information and white matter structures
in prefrontal cortex linked to processing speed [52, 53]. So, impairment of WM
leads to difficulties in manipulating information [16,54].
There are differences between the activity of brain area related to WM and
the level of function linked to used amount of WM resources in older adults
and younger adults, they have similar function but more activity of brain area
at low used amount of WM resources in older adults, and decline function
with less activity of brain area at high used amount of WM resources in older
adults [55-59].
Note, because of the fundamental individual differences in age-related
changes of the brain, there are considerably individual differences in the
difficulties caused by WM impairment on older adults everyday performance.
WM and CAPD
WM is one of the skills of central auditory processing and there is a direct
relationship between WM capacity and auditory processing function. The
High WM capacity lead to better auditory processing function and the low WM
capacity causes decline of auditory processing function [60]. The WM capacity
defined as the number of objects that a person can maintain in memory for
a short duration of time at the same time [61,62]. The WM capacity has a lot
of relation to reading comprehension and also intellectual effort of person
for learning new information [63,64]. The WM capacity limited in aging and
decline in the function of dorsolateral prefrontal cortex is responsible for this
limitation [65-67]. There is a difference in WM capacity between individuals,
this difference attributed to higher-level cognitive functions such as attention
and a large kind of complicated daily tasks [68-70]. The WM capacity is much
related to center of attention, maximum WM capacity is usually four items, if
more items are presented then center of attention stored them inside of longterm
memory [71,72].
Speech-in-noise (SIN) perception seems to rely on WM, since the fundamental
task of WM is discriminating between target message (speech) and untargeted
message (noise) and also larger WM capacity with involved of active attention
mechanism lead to better speech perception in noisy situation, So WM
decline lead to SIN perception faces the challenge in aging [73-76]. Ignoring
the untargeted message manages by inhibitory mechanism of WM and this
mechanism delayed in time in older adults [77,78].
In other hand, this time delay is due to age-depended changes that happen
in frontal cortex, particularly the prefrontal cortex [79,80]. Decrease in white
matter may effect on neural tracts that is associated with declined neural
conduction and processing speed [81,82].
In difficult auditory situation whether due to hearing loss or distracting
message, verbal WM has an important role because we become dependent
to visual information related to speech for better speech perception in this
situation [84-85].
Older adults in comparison to younger benefit more of context information
for speech perception and verbal WM is basic for this task. Considering the
importance of context information in aging and also age-sensitive of WM for
context, whether hearing thresholds are normal or not, most of the old adults
have speech perception problems [86].
The auditory system has two distinct categories, the peripheral auditory
system (outer ear, middle ear and inner ear) and the central auditory system
that compose of auditory pathway in the central nervous system (auditory
nerve, brain stem and auditory areas in the cortex). The peripheral auditory
system tasks are reception of sound stimulus; transform the stimulus to the
electrical signals that transmit by the auditory nerve to the higher auditory
centers for decoding and perception of the information [87].
The age-related hearing loss (presbycusis) that attributed to the peripheral
auditory system impairment lead to decline of hearing thresholds [88-90],
but in the central auditory impairment that is known as central auditory
processing disorder (CAPD) the hearing thresholds are normal but there is
a difficulty in speech perception especially under complicated listening
position such as noisy environments. The SIN perception skill related to the
central auditory system [91]. Cognitive process is very essential for speech
perception especially when listening situation exposed to noise or any other
competing message [92-93] because noise or any unwanted sound can impair
your memorization skills and also WM performance. There is a great link
between attention and executive function component of WM and CAPD, so
that individuals with CAPD often diagnosed with impairment of attention and
executive functioning of WM Figure 2.
Figure 2: Relation between central executive segment of WM and CAPD.
WM and Music
Many cognitive functions including working memory impaired in Alzheimer's
disease (AD) and cognitive impairment apparent in the early stage of AD [94].
The Center for Disease Control has reported that AD is the sixth main reason
of death in the United States among people above the age of 65 years.
The evidences demonstrated that cognitive abilities are impacted by music
[95,96]. Listening to music affects our behavior, activities and emotional
state. Music has positive effects on mood and communication all over
the life especially during aging. The effects of music on memory tasks
related to its effects on mood, psychic and emotion. One of the destructive
processes in neurodegenerative disease that involved memory such as
Alzheimer's disease is atrophy and decrease in number of neurons in the
brain areas especially in the hippocampus. One study showed that music
has the positive effects on short term memory in the autistic rat pups [97].
Functional magnetic resonance imaging (fMRI) showed that Cognitive
training increased activity in the prefrontal and parietal [98]. Studies have
shown that WM training progress the rate of processing and some skills
of the central auditory processing such as attention, language perception
and cognitive control [99].
Classical music has positive effect on spatial reasoning. However individual
variables such as mood, the arousal level and musical interest strongly linked
with effects of music [100]. Music has indirect effect on learning and cognitive
functions by influence on mood, emotional state and arousal. Cheerful and
sweet music that person prefer to listen lead to better cognitive functions
[101]. Music is a complex acoustic stimulus with features such as melody,
timbre and perception of music is associated with syntactic and semantic
processing, attention and working memory. Listen to the music entertain most
parts of the brain such as frontal, Temporal, cerebellar, parietal and limbic/
paralimbic [102]. Any functions related to music whether listening or singing
can contribute to decrease of stress through increase of social activity and
welfare among older adults [103].
Conclusion
Musicians have better performed in central auditory processing tasks such as
speech perception in noisy environments. And working memory is one of the
skills that necessary for listening to speech in noise. Therefore, according to
the role of working memory in central auditory processing and understand the
positive effects of music on working memory, this review can probably lead us
toward setting up the programs with purpose increase of speech perception
in older adults with central auditory processing disorder and also, evaluated
the central auditory system such as SIN perception is very important in older
adults.
Author Contributions
F.B contributed to the design and implementation of the research, to the
analysis of the results and to the writing of the manuscript.
Conflicts of Interest
The author declares no potential conflict of interest on publishing this paper.
Disclosure Statement
The author received no financial support for the research.
Acknowledgements
None.
References
- Weng, W., et al. The transfer effects of cognitive training on working memory among Chinese older adults with mild cognitive impairment: a randomized controlled trial. Front Aging Neurosci 11 (2019): 212.
- Goghari, V. M., & Lawlor-Savage, L. Comparison of cognitive change after working memory training and logic and planning training in healthy older adults. Front Aging Neurosci 9 (2017): 39.
- Nittrouer, S., et al. Verbal working memory in older adults: The roles of phonological capacities and processing speed. J Speech Lang Hear Res 59. 6 (2016): 1520-1532.
- Degé, F., & Kerkovius, K. The effects of drumming on working memory in older adults. Ann N Y Acad Sci 1423 (2018): 242-250.
- Teixeira-Santos, A. C., et al. Reviewing working memory training gains in healthy older adults: A meta-analytic review of transfer for cognitive outcomes. Neurosci Biobehav Rev (2019): 163-177.
- Weicker, J., et al. WOME: Theory-based working memory training—A placebo-controlled, double-blind evaluation in older adults. Front Aging Neurosci 10 (2018): 247.
- Fournet, N., et al. Evaluating short-term and working memory in older adults: French normative data. Aging Ment Health 16. 7 (2012): 922-930.
- Lugtmeijer, S., et al. A comparison of visual working memory and episodic memory performance in younger and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 26.3 (2019): 387-406.
- Di Rosa, E., et al. Reward motivation and neurostimulation interact to improve working memory performance in healthy older adults: A simultaneous tDCS-fNIRS study. Neuroimage 202 (2019): 116062.
- Reinhart, R. M., & Nguyen, J. A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci 22. 5 (2019): 820-827.
- Filbey, F. M., et al. Failing compensatory mechanisms during working memory in older apolipoprotein E-ε4 healthy adults. Brain Imaging Behav 4. 2 (2010): 177-188.
- Yang, H.-L., et al. (2019). Development and effectiveness of virtual interactive working memory training for older people with mild cognitive impairment: a single-blind randomised controlled trial. Age Ageing 48. 4 (2019): 519-525.
- Kirova, A.-M., et al. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Res Int (2015): 748212.
- Nissim, N. R., et al. Effects of transcranial direct current stimulation paired with cognitive training on functional connectivity of the working memory network in older adults. Front Aging Neurosci 11 (2019): 340.
- Saliasi, E., et al. Neural correlates associated with successful working memory performance in older adults as revealed by spatial ICA. PloS one 9. 6 (2014): e99250.
- Fostick, L., & Revah, H. Dyslexia as a multi-deficit disorder: Working memory and auditory temporal processing. Acta Psychol 183 (2018): 19-28.
- Leenders, M. P., et al. Diminished alpha lateralization during working memory but not during attentional cueing in older adults. Cereb Cortex 28 (2018): 21-32.
- Ballesteros, S., et al. Effects of video game training on measures of selective attention and working memory in older adults: results from a randomized controlled trial. Front Aging Neurosci 9 (2017): 354.
- Mičič, S., et al. The Impact of Working Memory Training on Cognitive Abilities in Older Adults: The role of Cognitive Reserve. Curr Aging Sci 13 (2020): 52-61.
- Vaqué-Alcázar, L., et al. Functional and structural correlates of working memory performance and stability in healthy older adults. Brain Struct Funct 225 (2020): 375-386.
- Borella, E., et al. Working memory training for healthy older adults: the role of individual characteristics in explaining short-and long-term gains. Front Hum Neurosci 11( 2017): 99.
- Woods, S. P., et al. Spontaneous strategy use protects against visual working memory deficits in older adults infected with HIV. Arch Clin Neuropsychol 25. 8 (2010): 724-733.
- Avila, R. T., et al. (2015). Working memory and cognitive flexibility mediates visuoconstructional abilities in older adults with heterogeneous cognitive ability. J Int Neuropsychol Soc 21. 5 (2015): 392-398.
- Nissim, N. R., et al. (2017). Frontal structural neural correlates of working memory performance in older adults. Front Aging Neurosci 8 (2017): 328.
- McAvinue, L. P., et al. An evaluation of a working memory training scheme in older adults. Front Aging Neurosci 5 (2013): 20.
- Brown, L. A., et al (2012). Processing speed and visuospatial executive function predict visual working memory ability in older adults. Exp Aging Res 38 (2012): 1-19.
- Curtis, A. F., et al. Improving visual spatial working memory in younger and older adults: effects of cross-modal cues. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 26(2019): 24-43.
- Zinke, K., et al. (2014). Working memory training and transfer in older adults: effects of age, baseline performance, and training gains. Dev Psychol 50 (2014): 304-315.
- Nicholls, L. A. B., & English, B. Multimodal coding and strategic approach in young and older adults’ visual working memory performance. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 27(2020): 83-113.
- Pliatsikas, C., et al. Working memory in older adults declines with age, but is modulated by sex and education. Q J Exp Psychol (Hove) 72. 6 (2019): 1308-1327.
- Bugg, J. M., et al. Physical activity moderates time-of-day differences in older adults' working memory performance. Exp Aging Res 32. 4 (2006): 431-446.
- Osaka, M., et al. Verbal to visual code switching improves working memory in older adults: an fMRI study. Front Hum Neurosci 6 (2012): 24.
- Andiel, C., & Liu, L. Working memory and older adults: Implications for occupational therapy. Am J Occup 49. 7 (1995): 681-686.
- Borella, E., et al. (2010). Working memory training in older adults: evidence of transfer and maintenance effects. Psychol Aging 25. 4 (2010): 767.
- Brehmer, Y., et al. Working-memory training in younger and older adults: training gains, transfer, and maintenance. Front Hum Neurosci 6 (2012): 63.
- Brum, P. S., et al. Working memory training format in older adults: individual versus group sessions. Aging Clin Exp Res 32. 11 (2020): 2357-2366.
- Gamboz, N., et al. The role of switching, inhibition and working memory in older adults' performance in the Wisconsin Card Sorting Test. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 16. 3 (2009): 260-284.
- Hara, Y., & Naveh-Benjamin, M. (2015). The role of reduced working memory storage and processing resources in the associative memory deficit of older adults: simulation studies with younger adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 22. 2 (2015): 129-154.
- Borella, E., et al. Are age-related differences between young and older adults in an affective working memory test sensitive to the music effects? Front Aging Neurosci 6 (2014): 298.
- Gerhardsson, A., et al. Positivity effect and working memory performance remains intact in older adults after sleep deprivation. Front Psychol 10 (2019): 605.
- López-Higes, R., et al. Efficacy of cognitive training in older adults with and without subjective cognitive decline is associated with inhibition efficiency and working memory span, not with cognitive reserve. Front Aging Neurosci 10 (2018): 23.
- Thurm, F., et al. Comparing effects of reward anticipation on working memory in younger and older adults. Front. Psychol 9 (2018): 2318.
- Gilchrist, A. L., et al. Working memory capacity for spoken sentences decreases with adult ageing: Recall of fewer but not smaller chunks in older adults. Memory 16. 7 (2008): 773-787.
- Goral, M., et al. The contribution of set switching and working memory to sentence processing in older adults. Exp Aging Res 37. 5 (2011): 516-538.
- Insel, K., et al. Executive function, working memory, and medication adherence among older adults. J Gerontol 61. 2 (2006): P102-P107.
- Heinzel, S., et al. Working memory training improvements and gains in non-trained cognitive tasks in young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 21. 2 (2014): 146-173.
- Berryhill, M. E., & Jones, K. T. tDCS selectively improves working memory in older adults with more education. Neurosci Lett 521. 2 (2012): 148-151.
- Boller, B., et al. Relationships between years of education, regional grey matter volumes, and working memory-related brain activity in healthy older adults. Brain Imaging Behav 11. 2 (2017): 304-317.
- Heinzel, S., et al. Working memory load-dependent brain response predicts behavioral training gains in older adults. J Neurosci 34. 4 (2014): 1224-1233.
- Salami, A., et al. Neurocognitive profiles of older adults with working-memory dysfunction. Cereb Cortex 28. 7 (2018): 2525-2539.
- Salat, D. H., et al. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cereb Cortex 12. 5 (2002): 494-505.
- Kawagoe, T., et al. Brain activation during visual working memory correlates with behavioral mobility performance in older adults. Front Aging Neurosci 7 (2015): 186.
- Rizio, A. A., & Diaz, M. T. Language, aging, and cognition: Frontal aslant tract and superior longitudinal fasciculus contribute to working memory performance in older adults. Neuroreport, 27. 9 (2016): 689.
- Heinzel, S., et al. Transfer effects to a multimodal dual-task after working memory training and associated neural correlates in older adults–a pilot study. Front Hum Neurosci 11(2017): 85.
- Hakun, J. G., & Johnson, N. F. Dynamic range of frontoparietal functional modulation is associated with working memory capacity limitations in older adults. Brain Cogn 118 (2017): 128-136.
- Heinzel, S., et al. Prefrontal-parietal effective connectivity during working memory in older adults. Neurobiol Aging 57 (2017): 18-27.
- Heinzel, S., et al. Neural correlates of training and transfer effects in working memory in older adults. NeuroImage 134 (2016): 236-249.
- Nagel, I. E. et al. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci 23. 8 (2011): 2030-2045.
- Oren, N., et al. Neural patterns underlying the effect of negative distractors on working memory in older adults. Neurobiol Aging 53 (2017): 93-102.
- Moossavi, A., et al. The relation between working memory capacity and auditory lateralization in children with auditory processing disorders. Int J Pediatr Otorhinolaryngol 78. 11 (2014): 1981-1986.
- Lotfi, Y., et al. Relation between working memory capacity and auditory stream segregation in children with auditory processing disorder. Iran J Med Sci 41. 2 (2016): 110.
- Rodríguez-Aranda, C., et al. Association between executive functions, working memory, and manual dexterity in young and healthy older adults: an exploratory study. Percept Mot Skills 122(2016): 165-192.
- Ehrlich, M.-F., et al. Working-memory capacity and reading comprehension in young and older adults. Psychol Res 56. 2 (1994): 110-115.
- Ganzer, C. A., et al. Associations between working memory, health literacy, and recall of the signs of stroke among older adults. J Neurosci Nurs 44. 5 (2012): 236-243.
- Bagheri, F., et al. Auditory Training Among Older Adults with Alzheimer disease and Central Auditory Processing Disorder. Avicenna J Neuro Psycho Physio 5. 4(2018).
- Bauer, E., et al. Performance level and cortical atrophy modulate the neural response to increasing working memory load in younger and older adults. Front Aging Neurosci 10 (2018): 265.
- Toril, P., et al. Video game training enhances visuospatial working memory and episodic memory in older adults. Front Hum Neurosci 10 (2016): 206.
- Bo, J., Jennett, S., & Seidler, R. Differential working memory correlates for implicit sequence performance in young and older adults. Exp Brain Res 221. 4 (2012): 467-477.
- Guye, S., & Von Bastian, C. C. Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychol Aging 32. 8 (2017): 732.
- Werkle-Bergner, M., et al.Inter-individual performance differences in younger and older adults differentially relate to amplitude modulations and phase stability of oscillations controlling working memory contents. NeuroImage 60(2012): 71-82.
- Basak, C., & O’Connell, M. A. To switch or not to switch: Role of cognitive control in working memory training in older adults. Front Psychol 7 (2016): 230.
- Verhaeghen, P., e al. Resolving age-related differences in working memory: Equating perception and attention makes older adults remember as well as younger adults. Exp Aging Res 45. 2 (2019): 120-134.
- Hayes, M. G., et al. Working memory and the strategic control of attention in older and younger adults. J Gerontol B Psychol Sci Soc Sci 68. 2 (2013): 176-183.
- Janse, E., & Jesse, A. Working memory affects older adults’ use of context in spoken-word recognition. Q J Exp Psychol (Hove) 67. 9 (2014): 1842-1862.
- Nagaraj, N. K. Working memory and speech comprehension in older adults with hearing impairment. J Speech Lang Hear Res 60. 10 (2017): 2949-2964.
- Wayne, R. V., et al. Working memory training and speech in noise comprehension in older adults. Front Aging Neurosci 8 (2016): 49.
- 77.Clapp, W. C., et al. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. PNAS 108. 17 (2011): 7212-7217.
- Pelegrina, S., et al. Similarity-based interference in a working memory numerical updating task. Exp Psychol 59. 4 (2012): 183-189.
- Gilchrist, A. L., et al. Retrospective cues based on object features improve visual working memory performance in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 23. 2 (2016): 184-195.
- 80.Jones, K. T., et al. Longitudinal neurostimulation in older adults improves working memory. PloS one 10. 4 (2015): e0121904.
- 81. Oberlin, L. E., et al. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. NeuroImage 131 (2016): 91-101.
- 82. Rast, P. (2011). Verbal knowledge, working memory, and processing speed as predictors of verbal learning in older adults. Dev Psychol 47. 5 (2011): 1490.
- 83. Arciniega, H., et al. Frontoparietal tDCS benefits visual working memory in older adults with low working memory capacity. Front Aging Neurosci. 10 (2018): 57.
- 84. Feld, J. E., & Sommers, M. S. Lipreading, processing speed, and working memory in younger and older adults. J Speech Lang Hear Res 2009.
- 85. Truong, L., & Yang, L. (2014). Friend or foe? Decoding the facilitative and disruptive effects of emotion on working memory in younger and older adults. Front Psycho 5 (2014): 94.
- 86. McCabe, J., & Hartman, M. (2008). Working memory for item and temporal information in younger and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 15. 5 (2008): 574-600.
- 87. Magimairaj, B. M., & Nagaraj, N. K. Working memory and auditory processing in school-age children. LANG SPEECH HEAR SER 49. 3 (2018): 409-423.
- 88. Baldwin, C. L., & Ash, I. K. Impact of sensory acuity on auditory working memory span in young and older adults. Psychol Aging 26 (2011): 85.
- 89. Doherty, K. A., & Desjardins, J. L. The benefit of amplification on auditory working memory function in middle-aged and young-older hearing impaired adults. Front Psychol 6 (2015): 721.
- 90. Frtusova, J. B., & Phillips, N. A. The auditory-visual speech benefit on working memory in older adults with hearing impairment. Front Psychol 7 (2016): 490.
- 91. Bagheri, F., et al. Alzheimer’s Disease and Hearing Loss among Older Adults: A Literature Review. JPBS 8. 5 (2018): 77-80.
- 92. Frtusova, J. B., et al. ERP evidence that auditory–visual speech facilitates working memory in younger and older adults. Psychol Aging 28. 2 (2013): 481.
- 93. James, P. J., et al. Working memory predicts semantic comprehension in dichotic listening in older adults. Cognition 133 (2014): 32-42.
- 94. Huntley, J., et al. Adaptive working memory strategy training in early Alzheimer's disease: randomised controlled trial. Br J Psychiatry 210 (2017): 61-66.
- 95. Borella, E., et al. Is working memory training in older adults sensitive to music? Psychol Res 83. 6 (2019): 1107-1123.
- 96. Tumuluri, I., et al. Effectiveness of music therapy on focused attention, working memory and stress in Type 2 diabetes: An exploratory study. Int J Yoga 10. 3 (2017): 167.
- 97. Lee, S.-M., et al. Music application alleviates short-term memory impairments through increasing cell proliferation in the hippocampus of valproic acid-induced autistic rat pups. J Exerc Rehabil 12. 3 (2016): 148.
- 98. Bergman Nutley, S., et al. Music practice is associated with development of working memory during childhood and adolescence. Front Hum Neurosci 7 (2014): 926.
- 99. Hyer, L., et al. Cognitive training program to improve working memory in older adults with MCI. Clin Gerontol 39. 5 (2016): 410-427.
- 100. Kuschpel, M. S., et al. Differential effects of wakeful rest, music and video game playing on working memory performance in the n-back task. Front Psychol 6 (2015): 1683.
- 101. Lehmann, J. A., & Seufert, T.The influence of background music on learning in the light of different theoretical perspectives and the role of working memory capacity. Front Psychol 8 (2017): 1902.
- 102. Särkämö, T., et al. Cognitive, emotional, and social benefits of regular musical activities in early dementia: randomized controlled study.Gerontologist 54. 4 (2014): 634-650.
- 103. Sakamoto, M., et al. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int Psychogeriatr 25. 5 (2013): 775-784.
Author Info
Fereshteh Bagheri*
Department of Audiology, School of Rehabilitation Sciences, Babol University of Medical Sciences, Mazandaran, Iran
Citation: Temitope SA&Musa VM. Effect of Bromelain on BDNF level and memory deficit following intra-medial forebrain bundle 6-OHDA injection in rat model of Parkinsonism. J NeurolNeurophy, 2020, 11(7), 509.
Received: 19-Aug-2020
Published:
18-Nov-2020, DOI: 10.35248/2155-9562.20.11.505
Copyright: 2020 Bagheri F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution, and reproduction in any medium, provided the original author and source are credited

