Case Report - (2025) Volume 11, Issue 2
Chronic Lymphocytic Leukemia (CLL) is the most prevalent leukemia in adults in western countries. Remarkable strides have been achieved in treatment of CLL. One notable class of agents revolutionizing CLL treatment is Bruton’s Tyrosine Kinase inhibitors (BTKi), which have reshaped the landscape by proving viable alternatives to traditional chemotherapy and immunotherapy. The BTKi are indicated both in relapsed/refractory and at first line setting patients. The first-in-class BTKi, Ibrutinib, is generally administered until disease progression or until unacceptable toxicity. Despite Ibrutinib is considered easy manageable and well tolerated drug, instances of discontinuation are not uncommon. The most frequent reason leading to Ibrutinib discontinuation are infections. Drug discontinuation could determine a quick arise in lymphocyte count and their migration in lymphoid organs, leading to a rapid progression of the disease, in terms of reappearing of general symptoms, rapidly worsening of lymphadenopathy ore splenomegaly. In real life settings it has been documented a rapid drop of lymphocyte count attributable to the migration of lymphocyte to the spleen. which could represent a potential cause for splenic infarction and rupture. We reported the case report of a patient who discontinued Ibrutinib due to pulmonary infection. Subsequently, the patient experienced a life-threatening episode of splenic rupture, underscoring the critical considerations surrounding the discontinuation of such targeted therapies in the management of CLL.
Chronic lymphocytic leukemia • Ibrutinib • Peri-centimetric • lymphonodes Computerized tomography
Chronic Lymphocytic Leukemia (CLL) is the most prevalent leukemia in adults in western countries. Over the past decade’s significant development was made in treatment strategies in CLL. Novel target agents, including Bruton’s Tyrosine Kinase inhibitors (BTKi), have changed the CLL treatment scenario without using chemotherapy and immunotherapy, indicated both in relapsed/ refractory and at first line setting patients. The first-in-class BTKi, Ibrutinib, irreversibly inhibits B-cell’s BTK intracellular pathway, impeding CLL cells migration and adhesion from peripheral blood to lymphoid tissues (leading to an expected transient early-phase therapy lymphocytosis), proliferation and interaction with tumor microenvironment. Treatment with Ibrutinib should be continued until disease progression or unacceptable toxicity. Despite Ibrutinib is considered easy to handle and well tolerated drug, discontinuing therapy is not so rare [1-6].
The most frequent reason leading to Ibrutinib discontinuation can be disease progression, Richter syndrome, atrial fibrillation, bleeding, infections and second malignancies.
The rate of discontinuation because of adverse events is most frequent during the first year of Ibrutinib treatment and generally decreased over time [7].
Serious infections are among the most frequent treatment related adverse events. Between infections, pneumonia is more common in relapsed/refractory cases than in treatment-naïve patients who had been started on Ibrutinib. However, several studies demonstrated that they can occur early on treatment after a median time of 7 months from starting Ibrutinib. This observation suggests careful monitoring of patients during the first months.
Ibrutinib discontinuation could determine a lack of control in chemokine release and lymphocytes proliferation, determining a quick arise in lymphocyte count and their migration in lymphoid organs. This could lead to a rapid disease progression or Richter’s transformation, in terms of reappearing of general [8-10].
Symptoms, rapidly worsening of splenomegaly or lymphadenopathy and progressively laboratory changes (rising lymphocyte count or lactate dehydrogenase values).
In real life settings it has been observed a rapid drop of lymphocyte count depending on lymphocyte spleen migration, which could represent a risk factor for splenic infarction and rupture [11-13].
The case report we present refers to a 74-years old man affected by CLL who presented acute splenic rupture after a temporarily cessation of Ibrutinib.
The patient had the history of prostatic cancer treated with surgery and radiotherapy in 2013, relapsed in 2020 and treated with hormonal therapy. During oncologic follow-up no splenomegaly or lymphocytosis were reported.
His hematological history started on June 10th, 2022, when he was admitted to the Emergency Room (ER), complaining of spread musculoskeletal pain. His blood investigations results were as follow: Hemoglobin (Hb) 9.5 gr/dl, White Blood Corpuscles (WBC) 115.000/mmc with lymphocyte 105.000/mmc, platelet count 181.000/mmc, Lactate Dehydrogenase (LDH) 322 UI/L, hepatorenal chemistry within normal limits, direct and indirect Coombs test were negative.
Computerized Tomography (CT) scan revealed splenomegaly with 25 cm of bipolar diameter, associated with sub and peri-centimetric lymphonodes above and below the diaphragm and confluent celiac and periaortic lymphonodes (dimensions 27 × 15 mm).
Peripheral blood smear showed characteristically small, mature appearing lymphocytes that raised 90% of the total cell count, several Gumprect’s nuclear shadows, few prolymphocytes. Flow cytometry confirmed the diagnosis of CLL 94% of CD5/CD19 (+) cells displaying the following phenotype: CD19 (+), CD5 (+), CD20 (+ +), CD23 (+), CD79b (+), CD49d (+) (99%), with kappa monoclonality. Cytogenetic analysis revealed the deletions of 13q14. NGS technique revealed TP53 disruption and Immunoglobulin Heavy Chain Variable (IGHV) region analysis demonstrates a not-mutated CLL clone.
In July, persisting symptomatic disease (abdominal pain, fever, fatigue) was associated with progressive lymphocytosis with an increase of >50% over 1 month’s period (WBC 257.000/mmc, L 240.000/mmc).
On August the 1st, therapy with Ibrutinib had been started. As we expected, lymphocytosis has trended steadily upwards to 375.000/ mmc, followed by a gradual decreasing trend to 150.360/mmc in September with improvement of patient’s clinical condition.
On October the 24th, patient presented an episode of nausea, vomiting and fatigue. Abdominal physical examination was negative. Sour vesicular murmur and basal crackling were detected at chest auscultation. Blood test, chest X-ray and test for COVID-19 were required and Ibrutinib was suspended until the receipt of the results in the suspicion of unset pneumonia.
On October the 26th the patient was admitted in ER for worsening of general malaise and fatigue. Chest’s CT scan revealed lobar pneumoniae and antimicrobial therapy with ceftriaxone and clarithromycin had started. Blood test results showed Hb 11.7 gr/dl, WBC 27.120/, lymphocyte 20.000/mmc, platelet count 86.000/mmc. On October the 30th a second ER admission was required for acute abdominal pain. Abdominal CT scan revealed splenic artery’s pseudoaneurysm, large ruptured of subcapsular splenic hematoma >50% and hemoperitoneum. Splenic artery embolization was performed. 24 h after the onset CT control showed increasing splenic volume with 80% of ischemic rate. Therefore, urgent splenectomy and partial pancreatectomy was demanding.
Pathological examination showed a spleen size of 24 × 17 × 8 cm and a 9 cm capsular laceration. The cut surface showed a whitish micronodular aspect. Microscopic analysis of splenic and omentum sections points out a splenic infiltration of small lymphocytes with some nucleates elements with larger nuclei. Immunohistochemistry revealed the following phenotype: CD20+, CD23+, CD21-, CD5+. Ciclin D1 e SOX11 tested negative.
After surgical procedure peripheral blood examination showed Hb 7.9 gr/dl, WBC 66.750/mmc with lymphocyte 55.800/mmc, platelet count 317.000/mmc.
On December the 1st Ibrutinib had been reintroduced at full dosage after the completion of antibiotics and complete recovery of the patient. After 1 month of Ibrutinib, WBC count has been showing a stable trend around 60.000/mmc with improvement in the patient’s clinical condition.
At 10 months of follow-up, patient is in good general condition carrying out his daily activities. Last blood test results showed improvement in hemoglobin value and stability of leukocyte value: Hb 15.6 gr/dl, WBC 67840/, lymphocyte 60560/mmc, platelet count 335.000/mmc (Figures 1-4).
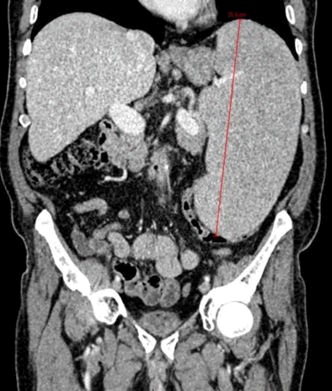
Figure 1. TC July 2022: Spleen 25 cm BP diameter.
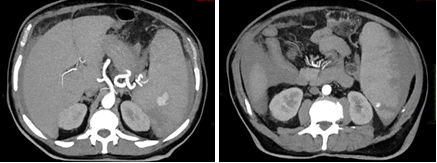
Figure 2. TC October 2022: Pseudoaneurysm and hemoperitoneum previous embolization.
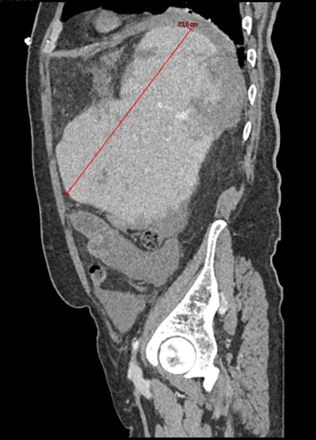
Figure 3. TC October 2022: Pseudoaneurysm and hemoperitoneum previous embolization.
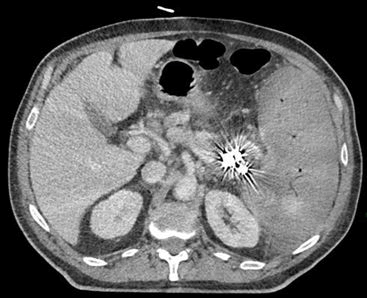
Figure 4. TC November 2022: After embolization and previous splenectomy: Splenomegaly with 80% of ischemic rate.
We reported the case of an elderly Treatment Naïve (TN) patient who discontinued Ibrutinib due to a pulmonary infection.
Many studies proved that toxicity, in particular infections, is the most common reason of Ibrutinib discontinuation.
CLLs is characterized by profound immune dysregulation, with enhanced susceptibility to infections. This could be explained by CLL cells inhibiting antibody production by plasma cells and inducing neutropenia by bone marrow involvement, reducing the amount of peripheral mature B-cells antibody, and down regulating antigens expression of T and natural-killer cells and more frequently to the effect of previous Immunochemotherapy received.
Immunoglobulin A (IgA) is the predominant antibody isotype in the mucosal immune system, which widely coats the gastrointestinal and respiratory tract. They have a fundamental role in protection against infections eliminating the pathogens with immune exclusion via nonspecific immunity. They represent the first line of defence against respiratory viruses.
Sun C, et al. observed, in a large cohort of patients during an extended 24 months’ follow-up, an increasing IgA levels within 6 month starting Ibrutinib [14]. IgG level remained stable in short term decreasing with longer duration of drug. Moreover, they observed that Ibrutinib decreased IgM levels in patient with clonal IgM, whereas increased IgM levels in patients without paraprotein.
Mato, et al. recently demonstrate in a large cohort of patients, both in and off clinical trials, that toxicity is the major reason of discontinuation both in the Relapsed/Refractory (R/R) and TN setting patients [15].
Differently, Susan O’Brien, et al. shows how R/R patients discontinued treatment most commonly because of disease progression [16].
Contrary of what we observed in this case report, O'Brien et al. showed that grade ≥ 3 infections occurred less frequently among TN patients compared with R/R patients [17].
Similarly, to what we described, in patients receiving Ibrutinib, the risk of infections was greatest during the first 3 months of therapy and decreased over time. Disease-related risk of infection seems to decrease following 6 months of treatment. This could be related to several reasons, including early responses and disease, reduction of immunosuppressive T cells, extended time from last chemotherapy (in the case of R/R patients), and the immune-modulating potential of Ibrutinib.
Most of clinical trials about Ibrutinib reported that respiratory infection represents the 40% of all the infections. Streptococcus pneumoniae and Haemophilus influenzae are major causal agents for pneumonia and both are encapsulated bacteria, for which antibody response is crucial. Over two decades, with extended use of Ibrutinib, show an increasing incidence of invasive aspergillosis and other fungi, including Pnemocystis jirovecii (PJ). PJ pneumonia in 5% of cases could manifest with atypical clinical presentation, leading to delay in diagnosis.
During infections of grade >3, temporarily Ibrutinib discontinuation is often recommended. As Paul J Hampela, et al. overserved in his study 25% of patient who temporarily discontinued Ibrutinib underwent to a progression of CLL disease or Richter’s transformation, manifesting worsening of symptoms and adenopathy, rising lymphocyte count and, how described both Swetha Paduri and Porpaczy E, et al. in their case reports, it could also result in a rapid enlargement and rupture of the spleen [18,19]. Porpaczy E, et al. hypotized that splenomegaly, before Ibrutinib is suspended, could represent a risk factor for this phenomenon. Differently, Dutton, et al. in his case report described a rapid drop of peripheral blood lymphocyte count followed by rapid increase of splenic size and his rupture, similarly to what we observed, hypnotizing that Ibrutinib discontinuation could determine systemic cytokines release and losing in lymphocyte proliferation and their homing control to most of lymphoid tissues [20]. Moreover, they hypotize that discontinuing Ibrutinib when lymphocyte count is still significantly high could represent a risk factor. Additionally, splenic rupture is also observed in other hematologic disease like R/R Mantle cell lymphoma in combined therapy Ibrutinib and venetoclax.
Only 17% of splenic rupture cases has typical clinical manifestation of acute abdomen hemoperitoneum. Sometimes, elderly age, infection conditions and disease symptoms could mask clinical presentation determining an important diagnostic delay.
In order to prevent dangerous complications, especially during the first year of treatment, Ibrutinib discontinuation should be avoided and restricted only for necessary cases, like serious infections, especially when intravenous antibiotic or antifungal therapy is suggested.
We recommend to be careful in Ibrutinib discontinuation in elderly patients who present lymphocytosis and splenomegaly.
For patient with severe splenomegaly, who necessitate Ibrutinib discontinuation, we recommend frequent blood count monitoring, focusing on lymphocytosis, to exclude that sudden reduction is not an expected effect of the therapy but rather an indicator of splenic sequestration following drug discontinuation. Furthermore, it is useful to perform ultrasound monitoring every 2-3 months and a weekly physical examination or in case of pain in the upper left abdominal quadrant or at the apex of the contralateral shoulder. We suggest to educate the patients about the possible consequences without alarming them.
Moreover, in presence of signs of disease progression following the discontinuation of Ibrutinib, it is always useful to perform a disease reassessment. For example, in case of splenectomy, it is advisable to supplement the histological examination of the spleen with immunohistochemical analysis to exclude Richter transformation.
As we explained above, hypogammaglobulinemia is an infection risk factor in particular for NT patients. We suggest that in this setting of patients monitoring immunoglobulin serum lever could be a guide for IV immunoglobulin replacement prophylaxis.
Over time, there has been growing recognition of the possible consequences when discontinuing Ibrutinib, along with valuable advice and warnings about exercising caution in such situations, including interruptions for medical procedures.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Luigi ATR. "Splenic Rupture after Ibrutinib Discontinuation". Oncol Cancer Case Rep, 2025, 11(1), 1-4.
Received: 22-Jan-2024, Manuscript No. OCCRS-24-125565; Editor assigned: 24-Jan-2024, Pre QC No. OCCRS-24-125565 (PQ); Reviewed: 07-Feb-2024, QC No. OCCRS-24-125565; Revised: 19-Mar-2025, Manuscript No. OCCRS-24-125565 (R); Published: 26-Mar-2025, DOI: 10.35248/2471-8556.25.11(1).001-007
Copyright: © 2025 Luigi ATR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.