Hypothesis - (2022) Volume 13, Issue 11
Sensory Spine-Necks may be Perceptible
David Hubbard*
*Correspondence:
David Hubbard, Neuroscience, Research Professor, California School of Professional Psychology, Alliant International University-San Diego,
United States,
Email:
Author info »
Abstract
Hypothesis: A sensation (e.g., the twinkle of a star in a night sky) arises in the opening of the spine-neck membrane of primary cortical sensory pyramidal neurons
Introduction
Beginning in the 1950’s, Barlow and others established that sensory neurons are responsible for sensations [1-9]. Mountcastle confirmed that sensory thresholds were similar in humans and other primates [10]. Libet, in his “time-on” studies, compared the duration of the neural activity and onset of “liminal” sensations [11]. He showed that sensations persist unfaded for 500 to 5000 ms, and that the threshold for sensory duration is approximately 200 ms [12].
A well-studied visual sensation is a phosphene. A phosphene appears as a flash of light. They can be elicited in all visual fields, demonstrate a precise location at a narrow visual angle of less than one degree, and discrete colors [13-17]. With a stimulus duration of 250 ms, the subject sees the same shapes each time, and different subjects see different shapes [36]. Phosphenes appear and disappear immediately when stimulation is turned on and off with tightly defined onset and duration [18-22].
Sensations Arise in Sensory Neurons in cortex.
Phosphenes can be elicited in mid-layer cortex pyramidal neurons by a single electrode spreading current less than 0.5 mm, capable of influencing as few as 50 cells. The sensory neuron and its cortical column are connected widely through brain activation [23 -37].
Cortical Sensory Neurons Respond in an “instant” (< 50 ms).
In cats, visual cortex neurons respond in approximately 35 ms, slightly faster for changes in luminance than contrast [38]. In rodents, the somatosensory cortex neurons respond to whisker deflection in 12 ms. The timing and duration of each step from external sensory receptor to the lick response at 317 ms has been studied by Carl Petersen’s team [39, 40]. A single whisker deflection activated a single neuron in a cortical column that contains the neuron’s soma, axon and complete dendritic arbor. Using voltage-sensitive dye imaging through a craniotomy window they showed that the excitation to a single stimulus was initiated in the column at 2 ms. GABA inhibition had no effect on this early response [41]. On the hand, NMDA receptor-dependent plasticity was induced within two minutes, supporting a role for spine necks in memory [42]. Direct cortical stimulation was achieved via uxta-cellular pipette voltage stimulation and was optimized with a stimulus duration of 200 ms [43-45].
Sensations last longer than an instant, they last for a “moment” (50-400 ms).
The Petersen team also identified a late and prolonged depolarization at 50-400 ms. This “late” depolarization occurred in trained animals only. Whisker deflections stimulated action potentials in the same and nearby cortical columns in the first 50 ms followed by a rapid decline to baseline in 200 ms. The late potential, on the other hand, started at 50 ms and was sustained for 400 ms. The authors proposed that the late component causally participates in the subjective “sensory percept” [46 -45].
Cortical sensory spine-necks respond for a “moment” (50-200 ms).
Unique outpouchings along the sensory dendrite called spines receive the axons from the thalamus (Fig. 1).
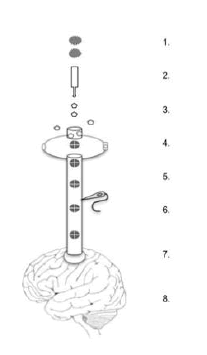
Figure 1: Peripheral Stimuli; 2. Thalamic Relay; 3. Glutamate; 4. Receptor. channels; 5. Positive ion influx; 6. Spine-neck EPSP with Electrode; 7. Dendrite; 8. Interneurons
In response to glutamate binding, the AMPA channel opens to positive ions initiating the depolarization along the membrane towards the dendrite. Nevertheless, the spine-neck potential does not significantly contribute to its own axon, leading to the proposal that spine-neck potentials are acting, not in the service of firing rates, but as an antenna system [29]. The spine neck’s length, diameter and shape are actively controlled by the actin skeleton that increases electrical resistance in the spine-neck membrane [47, 48, 49, 50, 51, 52]. The spine-neck resistance prolongs the membrane potential to 50-200 ms, longer with continuing external stimulation [53, 54,55, 56, 45, 57]. The spine-neck and axon share the same membrane, but the spine-neck potential lasts dramatically longer [6, 5].
Sensations may arise in cortical sensory spinenecks
Sensory spine-neck membrane potentials have precise spatial and temporal coordinates. One or a few may create a phosphene or may correlate with the smallest sensations. The adjacent NMDA receptors may save spine-neck potential patterns as memories. In the present moment the external environment is experienced by the individual during the same moment that the extracellular environment is open for (positive) ions entering the cell.
Phosphenes are sensations
Phosphenes are flashes of light evoked by stimulating the visual system mechanically or electrically. Phosphenes are typically small (less than 0.5 degrees of visual angle), round or complex patterns, and a variety of colors. They can be elicited in central and peripheral vision [13, 16, 58, 15, 17]. When a phosphene-eliciting stimulation duration of 250 ms is repeated, the subject sees the same shapes each time, different subjects see different shapes [18]. Phosphenes appear and disappear immediately when stimulation is turned on and off [19, 20, 30, 21, 23].
Phosphenes arise in primary visual cortex (V1) pyramidal neurons
Phosphenes can be elicited in primary visual cortex mid-layer pyramidal neurons by a single electrode spreading current less than 0.5mm [58, 36, 37, 38, 39, 40, 59,41, 42, 43, 44, 45]. In a mm2 of the human visual cortex there are 200,000 neurons and 3,084 billion (x109 ) synapses [60]. The search for a psychophysical mechanism has been focused on the fast-firing neural circuitry not the slow subcellular membrane potentials [46, 47, 49].
Synaptic spines on V1 dendrites have long, high resistance neck membranes
The V1 pyramidal neurons receive synaptic connections via the thalamus from the retina onto the dendritic spines. The postsynaptic membrane holds the AMPA- and NMDA- receptors for the primary excitatory transmitter (glutamate). The NMDA receptors modulate recall of those spine- necks [61].
The spine neck’s length, diameter and shape are controlled by the actin skeleton molecules that dramatically increase electrical resistance in that section of membrane [47, 48, 49,51, 52].
Spine neck membrane depolarizations are prolonged to 20-200 ms
From the synapse, the membrane depolarization spreads into the spine neck. The high electrical resistance in the spine neck prolongs the membrane potential from a few milliseconds to 20 to 200 milliseconds and longer with continuing external stimulation (53, 54, 55, 56, 45, 57).
Sensory membranes ‘late component’
Since Barlow’s studies in the 1950’s, it is well-established that sensory membranes areresponsible for subjective sensations [1, 8, 2, 3, 7, 9]. Sensory thresholds are the same for humans and macaques [10]. Activity of specific peripheral receptors gives rise to specific elementary sensations [4]. Libet found that the sensory threshold was about 200 ms and that sensation persisted unfaded from about 50 ms to 5000 ms [12]. In his “TimeOn” studies, he experimentally determined the correlation between the liminal sensation and the duration of neural activity [11]. Petersen and his colleagues have demonstrated that in response to whisker stimulation, mouse cortex barrel neurons respond with a “late component” that occurs 40-500 ms after stimulation; they propose that this late component causally participates in the subjective sensory percept [57, 58].
Hypothesis :
V1 spine-neck membrane potential durations of 50-200 ms will correlate with visual stimulation and subject self-report. Visual cortex spineneck membrane potentials are perceptible because they linger beyond the threshold for detection of about 50 ms. When the sensory membrane remains polarized for more than 50 ms, a sensation is experienced in that present moment. External stimulation provides the sufficient succession of synaptic EPSPs to sustain the spine-neck in a prolonged depolarization. In lay terms, when you see the smallest twinkling star, one spine-neck in your is responding. When you see more, many spinenecks are responding. The spine-necks respond not just for an instant but for a ‘split second’ (200-300 ms), the flap of a bird’s wing, and until the external stimuli change and the constellation of spinenecks change. Some such constellations we remember.
Contribution to the field
Dendritic spine-neck EPSPs are extraordinarily long in duration and unexplained. The spine-neck theory proposes that when the primary sensory neuron membrane at the spine neck is depolarized for an sustained duration, it is perceptible
References
- Barlow , H.B. “Single units and sensation: a neuron doctrine for perceptual psychology?”. Perception. 1.4(1972):371-394.
[Google scholar] [Crossref]
- Vallbo ÅB, Olsson KÅ, Westberg KG, Clark FJ. Microstimulation of single tactile afferents from the human hand: Sensory attributes related to unit type and properties of receptive fields. Brain. 1984 ;107(3):727-49. Google scholar
- Barlow H. The neuron doctrine in perception. Google scholar Crossref
- Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annual review of neuroscience. 1998;21(1):227-77. Google scholar Crossref
- Pessiglione M, Petrovic P, Daunizeau J.Subliminal instrumental conditioning demonstrated in the human brain. Neuron. 2008 ;28;59(4):561-7.Google scholar Crossref
- Brooks SJ, Savov V, Allzén E et al . Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. NeuroImage. 2012;59(3):2962-73.Google scholar Crossref
- Colombetti G, Lenci F. Membranes and sensory transduction. Springer Science & Business Media; 2012. Google scholar Crossref
- Johnson L. A, Wander J. D, Sarma et al. Direct electrical stimulation of the somatosensory cortex in humans using electrocorticography electrodes: a qualitative and quantitative report. Journal of neural engineering, 2013; 10(3) Google scholar Crossref
- Fain, G. L. (2019). Sensory transduction. Oxford University Press Google scholar
- Mountcastle VB, LaMotte RH, Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. Journal of neurophysiology. 1972;35(1):122-36.Google scholar Crossref
- Libet B. Reflections on the interaction of the mind and brain. Progress in neurobiology. 2006 1;78(3-5):322-6.Google scholar Crossref
- Libet B, Gleason CA, Wright EW et al. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). In Neurophysiology of consciousness. Birkhäuser, Boston, MA. 1993 ;249-268Google scholar Crossref
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. The Journal of physiology. 1968;196(2):479-93.Google scholar Crossref
- Tehovnik EJ, Tolias AS, Sultan F. Direct and indirect activation of cortical neurons by electrical microstimulation. Journal of neurophysiology. 2006;96(2):512-21.Google scholar Crossref
- Tehovnik EJ, Slocum WM, Smirnakis SM et al. Microstimulation of visual cortex to restore vision. Progress in brain research. 2009 ;175:347-75.Google scholar Crossref
- Tehovnik EJ, Slocum WM. Phosphene induction by microstimulation of macaque V1. Brain research reviews. 2007;53(2):337-43.Google scholar Crossref
- Lowery AJ, Rosenfeld JV, Lewis PM et al. Restoration of vision using wireless cortical implants: The Monash Vision Group project. In2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2015 ; 1041-1044.Google scholar Crossref
- Luo YH, Zhong JJ, Clemo M, da Cruz L. Long-term repeatability and reproducibility of phosphene characteristics in chronically implanted Argus II retinal prosthesis subjects. American journal of ophthalmology. 2016; 170:100-99.Google scholar Crossref
- Dobelle WH, Mladejovsky MG. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. The Journal of physiology. 1974;243(2):553-76.Google scholar Crossref
- Cattaneo Z, Vecchi T, Pascual‐Leone A, Silvanto J. Contrasting early visual cortical activation states causally involved in visual imagery and short‐term memory. European Journal of Neuroscience. 2009;30(7):1393-400.Google scholar Crossref
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends in cognitive sciences. 2008 ;12(12):447-54.Google scholar Crossref
- Silvanto J, Muggleton NG, Cowey A. Neural activation state determines behavioral susceptibility to modified theta burst transcranial magnetic stimulation. European journal of neuroscience. 2007;26(2):523-8.Google scholar Crossref
- De Graaf TA, Van den Hurk J, Duecker F et al. Where are the fMRI correlates of phosphene perception?Frontiers in neuroscience. 2018 ;12:883.Google scholar Crossref
- Schmidt EM, Bak MJ, Hambrecht FT et al . Feasibility of a visual prosthesis for the blind based on intracortical micro stimulation of the visual cortex. Brain. 1996; 119(2):507-22.Google scholar Crossref
- Delbeke J, Oozeer M, Veraart C. Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vision research. 2003;43(9):1091-102.Google scholar Crossref
- Chen , S.C ., et al. “Simulating prosthetic vision: I. Visual models of phosphenes”. Vis Res. 49.12(2009):1493-1506 .Google Scholar Crossref
- Lewis , PM et al. “Advances in implantable bionic devices for blindness: a review”. ANZ j. surg. 86.9(2016):654-9. Google Scholar Crossref
- Winawer J and Parvizi J. “Linking electrical stimulation of human primary visual cortex, size of affected cortical area, neuronal responses, and subjective experience”. Neuron. 92.6(2016):1213-9. Google Scholar Crossref
- Bosking, WH., et al. “Electrical stimulation of visual cortex: relevance for the development of visual cortical prosthetics”. Annu. rev. vis. sci. 5.3(2017):141. Google Scholar Crossref
- Self , MW., et al. “The segmentation of proto-objects in the monkey primary visual cortex”. Curr. Biol. 29.6(2019):1019-29. Google Scholar Crossref
- Fernandez, E., et al. “Development of a cortical visual neuroprosthesis for the blind: preliminary results”. Investig. Ophthalmol. Vis. Sci. 60.9(2019):4021. Google Scholar
- Fernández, E., et al. “Toward long-term communication with the brain in the blind by intracortical stimulation: Challenges and future prospects”. Front. neurosci. (2020):681. Google Scholar Crossref
- Farnum A and Pelled G. “New vision for visual prostheses”. Front. neurosci. (2020):36. Google Scholar Crossref
- Beauchamp, MS., et al. “Dynamic stimulation of visual cortex produces form vision in sighted and blind humans”. Cell. 181.4(2020):774-83. Google Scholar Crossref
- Ashtari, M., et al. “fMRI of retina-originated phosphenes experienced by patients with Leber congenital amaurosis”. PloS one.9.1(2014):e86068. Google Scholar Crossref
- Caparelli, EC., et al. “Simultaneous TMS-fMRI of the visual cortex reveals functional network, even in absence of phosphene sensation”. Open neuroimaging j. 4(2010):100. Google Scholar Crossref
- Caparelli, EC et al. “Simultaneous transcranial magnetic stimulation and functional magnetic resonance imaging: aspects of technical implementation”. Front. neurosci. 14(2020):554714. Google Scholar Crossref
- Terhune, DB., et al. “Phosphene perception relates to visual cortex glutamate levels and covaries with atypical visuospatial awareness”. Cereb. cortex. 25.11(2015):4341-50. Google Scholar Crossref
- Wang, WL., et al. V1 neurons respond to luminance changes faster than contrast changes. Scientific Reports. 5.1(2015):1-3. Google Scholar Crossref
- Petersen, CC., et al. Toward Biophysical Mechanisms of Neocortical Computation after 50 Years of Barrel Cortex Research. Function. 2.1(2021):zqaa046. Google Scholar Crossref
- Petersen, CC., The functional organization of the barrel cortex. Neuron. 56.2(2007):339-55.
- Petersen, CC. and Sakmann B. “Functionally independent columns of rat somatosensory barrel cortex revealed with voltage-sensitive dye imaging”. J. Neurosci. 21.21(2001):8435-46. Google Scholar Crossref
- Hwang, YS., et al. “3D Ultrastructure of synaptic inputs to distinct GABAergic neurons in the mouse primary visual cortex”. Cereb. Cortex. 31.5(2021):2610-24. Google Scholar Crossref
- Petersen CC, and Sakmann B. “The excitatory neuronal network of rat layer 4 barrel cortex”. J. Neurosci. 20.20(2000):7579-86. Google Scholar Crossref
- Petersen, CC., et al. “Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions”. J. Neurosci. 23.4(2003):1298-309. Google Scholar Crossref
- Yamashita T and Petersen CC. Target-specific membrane potential dynamics of neocortical projection neurons during goal-directed behavior. Elife. 21.5(2016):e15798. Google Scholar Crossref
- Sachidhanandam, S., et al. “Membrane potential correlates of sensory perception in mouse barrel cortex”. Nat. neurosci. 16.11(2013):1671-7. Google Scholar Crossref
- Romo, R., et al. “Somatosensory discrimination based on cortical microstimulation”. Nature. 392.6674(1998):387-90. Google Scholar Crossref
- Ray PG, et al. “Physiology of perception: cortical stimulation and recording in humans”. Neurology. 52.5(1999):1044 Google Scholar Crossref
- Yuste R, and Bonhoeffer T. “Morphological changes in dendritic spines associated with long-term synaptic plasticity”. Annu. rev. neurosci. 24.1(2001):1071-89. Google Scholar
- Ballesteros, Yáñez I., et al. “Density and morphology of dendritic spines in mouse neocortex”. Neuroscience. 138.2(2006):403-9. Google Scholar Crossref
- Oga T, et al. “Basal dendrites of layer-III pyramidal neurons do not scale with changes in cortical magnification factor in macaque primary visual cortex”. Front. Neural Circuits. 10(2016):74. Google Scholar Crossref
- Keliris GA, et al. “Estimating average single-neuron visual receptive field sizes by fMRI”. Proc. Natl. Acad. Sci. 116.13(2019):6425-34. Google Scholar Crossref
- Radman, T., et al. “Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro”. Brain stimul. 2.4(2009):215-28. Google Scholar Crossref
- Palmer LM and Stuart GJ. “Membrane potential changes in dendritic spines during action potentials and synaptic input”. J. Neurosci. 29.21(2009):6897-903. Google Scholar Crossref
- Harnett, MT., et al. “Synaptic amplification by dendritic spines enhances input cooperativity”. Nature. 491.7425(2012):599-602. Google Scholar Crossref
- Chen, H., et al. “A deep learning approach to automatic teeth detection and numbering based on object detection in dental periapical films”. Sci. rep. 9.1(2019):1-1. Google Scholar Crossref
- Staiger JF and Petersen CC. “Neuronal circuits in barrel cortex for whisker sensory perception”. Physiol. rev. 101.1(2021):353-415. Google Scholar Crossref
- Colonnier M and O'Kusky J. “Number of neurons and synapses in the visual cortex of different species”. Rev. can. biol. 40.1(1981):91-9. Google Scholar
- DeFelipe J. “The dendritic spine story: an intriguing process of discovery”. Front. neuroanat. 5.9(2015):14. Google Scholar Crossref
- Araya, R., et al. “Activity-dependent dendritic spine neck changes are correlated with synaptic strength”. Proc. Natl. Acad. Sci. 111.28(2014):E2895-904. Google Scholar Crossref
Author Info
David Hubbard*
Neuroscience, Research Professor, California School of Professional Psychology, Alliant International University-San Diego, United States
Citation: Hubbard D. Sensory Spine-Necks May Be Perceptible.j Neurol Neurophysiol. 2022, 13 (11), 001-003
Received: 04-Nov-2022, Manuscript No. jnn-22-78998;
Editor assigned: 05-Nov-2022, Pre QC No. jnn-22-78998(PQ);
Reviewed: 16-Nov-2022, QC No. jnn-22-78998 (Q);
Revised: 19-Nov-2022, Manuscript No. jnn-22- 78998 (R);
Published:
30-Nov-2022, DOI: 10.35248/2471-268X.22.13.11.605
Copyright: ©2022; Hubbard D. This is an open-access article distributed under the terms of the Creative Commons Attributio;]]]]]lon
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.