Mini Review - (2021) Volume 0, Issue 0
Sclerosing polycystic adenosis or adenoma- the term is open for discussion. The lesion is termed sclerosing polycystic adenosis-a pseudo neoplastic reactive inflammatory process that appears similar to sclerosing adenosis of the breast- in the recent WHO 2017 salivary gland tumour classification. It is categorised under other epithelial lesions as sclerosing polycystic adenosis and considers sclerosing polycystic adenoma as a synonym. The evidence to state the lesion to be a neoplastic process rather than reactive is on the rise and consideration should be given based on the degree of dysplasia that it presents, 30% local recurrence rate and genetic alteration related to PI3K pathway and PTEN, a tumour suppressor gene. The major portion of the ductal proliferations retains the lobular architecture and is lined by a layer of myoepithelial cells with rare incidence of invasive carcinoma. These features suggest the lesion to be considered a benign process until a rare invasive component or metastases is detected.
Sclerosing polycystic adenosis • Sclerosing polycystic adenoma • Apocrine differentiation • Hyalinization • Ductal carcinoma in situ • PI3K pathway • X-chromosome inactivation
The Sclerosing Polycystic Adenosis (SPA) was first described by Smith and colleagues in 1996 as a series of 9 cases from Armed Forces Institute of Pathology archives [1]. Till date around 100 cases have been documented in the English literature [2]. Around 70% of cases present in the parotid gland followed by submandibular gland (8%) and minor salivary glands (around 16%) [2-4]. Few cases have been reported in Sino nasal mucosa (3.2%) and lacrimal gland [2,3]. Familial occurrence of SPA in sisters has been documented where the younger sibling had multiple recurrences [5,6]. Female predominance is noted with the ratio of around 1.3:1 with a wide age range of 7-84 years. It presents as a slow growing mass with occasional pain. Multifocal presentation is observed [2,3,5,7]. On ultrasound, SPA is detected as hypoechoic, well-circumscribed lesion and on magnetic resonance imaging a T1 hypo signal and T2 hyper signal, well-circumscribed lesion distinct from adjacent parenchyma is noticed. In all conditions, imaging studies suggests a benign process without involvement of facial nerve [8-10]. Cytological aspirations of the lesion are non-diagnostic in most of the cases, mainly because of myriad of cytological presentation and rarity of the lesion. Predominantly, sheets of epithelial cells with apocrine metaplasia and sebocyte-like vacuolated cells when detected are suggestive of SPA. In case the epithelial cells present features of dysplasia in the setting of benign imaging studies, the suspicion is stronger for SPA. The other features noticed are background chronic inflammatory cells such as lymphocytes, plasma cells and foamy macrophages, oncocytic cells and squamous cells. In most cases, the initial cytological diagnosis is a benign salivary gland tumour such as pleomorphic adenoma, in cases where dysplastic features are marked mucoepidermoid Carcinoma (MEC) is suggested [10,11]. Although no known etiology has been reported, few cases have been reported after exposure to radiation, Epstein-Barr viral infection, and associated with lymphangioma [3,12]. The lesion is an isolated pathology in most cases. There are also reports of it occurring concurrently with pleomorphic adenoma, oncocytoma, Warthin’s tumour and sebaceous lymph adenoma [11]. A well circumscribed firm rubbery mass that may be multinodular is the macroscopic presentation. The cut surface shows pale areas, glistening areas and cystic spaces [1,6].
Under the microscope, a well circumscribed lobulated mass which may show partial or complete pseudo-capsulation is detected. It is mainly composed of dense hyalinization in nodules or plaques admixed with ductal proliferations and cystic changes (Figure 1). This appearance correlates well with fibrocystic sclerosing adenosis of the breast and thus the name sclerosing polycystic adenosis [4]. On closer examination, the ducts of varying sizes show lining epithelium exhibiting proliferation of epithelial cords bridging with each other, cribriform pattern surrounded by basement membrane like material, intraluminal papillary projections and solid proliferation of epithelial cells within the lumen are seen (Figure 2). Few cases show “roman bridge” pattern [5,12]. The epithelial cells proliferate in trabecular and tubuloductal patterns as well. The ductal Lumina may contain Periodic Acid Schiff (PAS) positive diastase resistant material and sometimes eosinophilic crystalloid material [12]. The other variations noted are flattening of ductal lining cells, lining cells being completely replaced by xanthomatous macrophages, and most importantly the ductal cells may show dysplasia of varying degree. The degree of dysplasia varies from low grade to high grade to ductal carcinoma in situ in around 40%-75% of cases [1,5,11,13]. Less than 10% of cases have reported high grade ductal carcinoma in situ [5,11]. The cystic component may be sparse in certain cases termed as paucicystic SPA [11]. The cells that line the ducts are cuboidal, columnar, squamous, mucous, sebaceous like cells (vacuolated cells), ballooned cells, clear cells, cells with apocrine sprouting and oncocytic cells. Atypical granular eosinophilic acinar cells, basophilic acinar cells with PAS positive diastase resistant cytoplasmic granules that resemble zymogen granules are evident in solid islands and microcytic lining (Figure 3) [2,11]. Lepromatous elements, hyaline globules of basement membrane, periductal stromal hyalinization, myxoid change, and chronic inflammatory infiltrate consisting of lymphoid follicles with germinal centers are the other components seen [2,11]. In certain conditions, the stromal proliferation may distort the ductal and acinar structures resembling an invasion. Stromal distortion may also lead to nodular scar tissue. Cribriform pattern of epithelial cells with nested basement membrane like material gives an appearance similar to collagenous spherulosis [1,11].
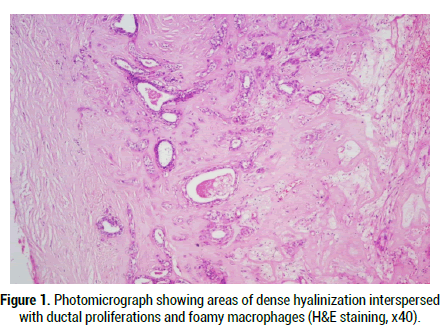
Figure 1: Photomicrograph showing areas of dense hyalinization interspersed with ductal proliferations and foamy macrophages (H & E staining, x40).
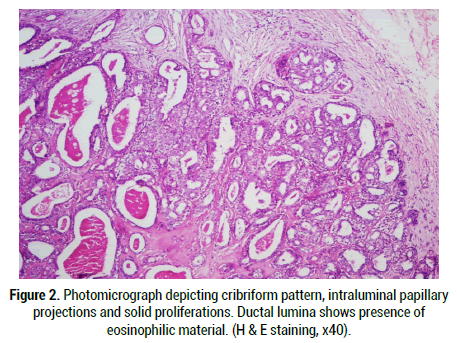
Figure 2: Photomicrograph depicting cribriform pattern, intraluminal papillary projections and solid proliferations. Ductal lumina shows presence of eosinophilic material. (H & E staining, x40).
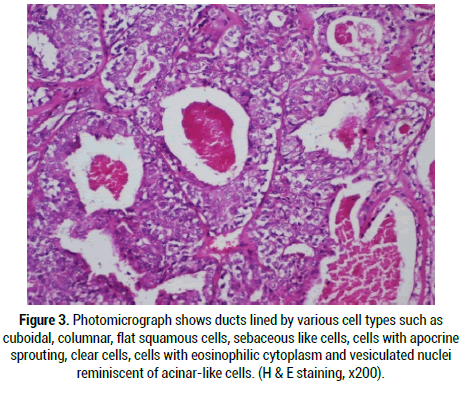
Figure 3: Photomicrograph shows ducts lined by various cell types such as cuboidal, columnar, flat squamous cells, sebaceous like cells, cells with apocrine sprouting, clear cells, cells with eosinophilic cytoplasm and vesiculated nuclei reminiscent of acinar-like cells. (H & E staining, x200).
Though a variation in pattern of arrangement of cells, cell types and dysplastic changes are evident; the lobules are surrounded by a layer of myoepithelial cells. This can be detected on myoepithelial markers such as calponin, p63, smooth muscle actin and muscle specific actin ascertaining that the lobular architecture is always preserved. Higher dysplastic grades (pleomorphism, central necrosis, high mitotic rate) when confined within the myoepithelial lining are termed as ductal carcinoma in situ [6,11]. The cells lining the lumen express cytokeratin, carcinoembryonic antigen, estrogen receptor [ER] (20%), and progesterone receptor [PR] (80%) [5,13]. The estrogen and progesterone receptors sometimes are absent in dysplastic areas whereas more than 90% of cells in cases of high grade ductal carcinoma in situ present with androgen receptor [AR] positivity in the nulecus [5]. The cells are positive for gross cystic fluid protein 15 (GCDFP-15) [2]. The proliferative (Ki-67) index is around 2% in epithelial cells and is increased in high grade ductal proliferations. One of the cases reported in labial minor salivary glands, high grade ductal proliferation was evident with a Ki-67 index of around 16% [5,11,14]. The study done by Skalova, et al. on PCR based analysis of X-chromosomal inactivation using Human Androgen Receptor (HUMARA) locus shows monoclonal nature of scelrosing polycystic adenosis cases [15]. The neoplastic nature of SPA is further supported by the study done by Bishop, et al. who have found genetic alterations related to phosphotidyl Inositol 3 Kinase (PI3K Pathway) with phosphatase and tensin homologue (PTEN), p110a subunit of PIK3 (PIKCA) and phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) mutations which are mainly tumour suppressor genes. Irrespective of ductal proliferations or presence of apocrine metaplasia, all the ductal and acinar cells showed mutations, but they were absent in myoepithelial cells [7]. A 30% local recurrence rate is documented that is related to multimodality and incomplete removal of the lesion [6]. The recurrences are late and may occur after 1.5 to 12 years of age [5]. A single case of invasive ductal carcinoma after repeated recurrences has been reported [4]. The differential diagnoses considered include polycystic digenetic disease, chronic sclerosing sialadenitis or Kuttner’s tumour, pleomorphic adenoma among the benign conditions. Bilateral presentation, occurrence in younger age group and the variations in the cell types separate this lesion from polycystic digenetic disease [2]. Absence of various cell types and lack of nodular pattern of hyalinization separates chronic sclerosing saialadenitis from SPA [1]. Lack of predominant myoepithelial cell growth patterns and acinar-like cells distinguishes SPA from pleomorphic adenoma [6].
Dysplastic features noted and focal acinar like cell proliferations prompts to distinguish it from acinar cell carcinoma. Confinement of dysplastic features within the ducts lined by myoepithelial cells and negativity for show positivity for Nuclear Receptor Subfamily 4 Group a Member 3 (NR4A3) differentiates SPA from actinic cell carcinoma. The cell of actinic cell carcinoma is positive for NR4A3 [1,2]. Prominent cystic component and papillary proliferations of the epithelial lining suggests low-grade cystadenocarcinoma to be a differential diagnosis. As against low-grade cystadenocarcinoma, the papillary proliferations are focal, there is variation in cell types, extensive sclerosis and lobules are lined by a single layer of myoepithelial cells [1, 6]. Presence of mucous, clear, cuboidal, columnar cells with cystic change and stromal inflammatory infiltrate points to consider MEC as a differential. Lack of an invasive component, prominent scar-like hyalinization and other cell types are the features that separate SPA from MEC [6]. High grade ductal carcinoma in situ cases present with comedo necrosis and increased mitoses that resemble salivary duct carcinoma. However, presence of myoepithelial lining the lobules of the lesional cells, lack of frank invasive component parts the lesion from salivary duct carcinoma [1]. The treatment followed is surgical excision in most cases. Care should be taken to completely remove the tumour, identifying even the multifocal presentation [3].
The lesion has been grouped under other epithelial lesions in the recent WHO 2017 salivary gland tumour classification with a speculation that only cases showing intraductal proliferations with dysplastic features may be neoplastic, the rest are just an inflammatory reactive process. Nevertheless, there are lot of features that suggests the condition to be neoplastic such as:
• The lesion shows progressive growth with cases presenting with various degrees of cytological atypia that is graded from low to high to ductal carcinoma in situ [4,10].
• Presence of apocrine differentiation in SPA cases which is a feature of high grade salivary duct carcinoma [7].
• Around 30% recurrence rate noted with multimodality [6].
• A case of invasive carcinoma after multiple recurrences documented [4].
• X-chromosome inactivation detected using polymorphism of the Human Androgen Receptor (HUMARA) [6,15].
• Monoclonal nature with genetic alteration in phosphotidyl inositol 3 kinase pathway (PI3K) with mutations mainly in PTEN gene that is detected in all ductal and acinar cells of SPA not just the proliferating regions [2,7].
Recognition of this lesion as neoplastic and ideally terming it as sclerosing polycystic adenoma is an imperative need. Identification of multifocal lesions and their management is essential to prevent recurrences especially as the recurrences are noted late in life suggesting a longer follow-up. Criteria to grade the dysplastic features when identified have to be setup to allow appropriate management. Possible expression of ER, PR and AR suggests a hormonal influence.
Citation: Venugopal R, Bavle RM, Muniswamappa S , Makarla S. Sclerosing Polycystic Adenosis/Adenoma-Changing Paradigm: A Mini Review. Med Rep Case Stud, 2021, 06(S4), 005-007
Received: 15-Sep-2021 Published: 06-Oct-2021, DOI: 10.35248/2572-5130.21.s4.005-007
Copyright: © 2021 Venugopal R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NO