Journal of Multiple Sclerosis
ISSN - 2376-0389NLM - 101654564
Research Article - (2025) Volume 11, Issue 4
Coronavirus 2019 (COVID-19) is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2) that mainly affects the lungs. COVID-19 symptoms include the presence of fevers, dry coughs, fatigue, sore throat, headaches, diarrhea and a loss of taste or smell. However, it is understood that SARS-CoV-2 is neurotoxic and neuro-invasive and could enter the central nervous system (CNS) via the hematogenous route or peripheral nerve route and causes encephalitis, encephalopathy, and acute disseminated encephalomyelitis (ADEM) in COVID-19 patients. This review paper discusses the possibility of SARS-CoV-2 mediated Multiple Sclerosis (MS) development in the future, a case comparable to the surge in Parkinson’s disease cases followed by the Spanish Flu in the year 1918. Moreover, the SARS-CoV-2 infection is associated with a cytokine storm. This paper highlights the impact of these modulated cytokines on glial cell interactions within the CNS, and its role in potentially prompting MS development.
Coronavirus • COVID-19 • SARS-CoV-2 • Multiple Sclerosis
On December 31st, 2019, Wuhan Municipal Health Commission in China reported a cluster of pneumonia cases, later named a respiratory disease coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Since then, the outbreak has crossed China’s borders and, later, a pandemic that affected more than 11.1 million people globally. The coronavirus infection is not new to the world. Within the past two decades, three significant coronavirus outbreaks have been identified – SARS (November 2002), MERS (June 2012), and COVID-19 (December 2019) [1]. Among these, SARS and COVID-19 caused by SARS and SARS-CoV-2 display similarities in amino acid sequence by 76% and has been shown to infect humans via the Angiotensin-Converting Enzyme 2 (ACE2) receptor. COVID-19 is the more infectious strain prompting countries to undergo lockdown with hampered trade, tourism, and education along with quick expansion in health care systems to adjust to the increased scale of infected individuals and fatalities with an average death rate of around 4.7%. Apart from the direct effect of SARS-CoV-2 on the lungs, these viruses tend to impact the central nervous system and hence the neuro-invasiveness and neurotropic nature of the SARS-CoV-2 needs to be considered. Interestingly, previous studies have shown an association of coronavirus with Multiple Sclerosis (MS). The Mouse Hepatitis Virus (MHV), a murine coronavirus-induced model is widely used in research to understand the demyelination mechanisms associated with MS. This review emphasizes the possible neuro-invasive route of SARS-CoV-2 and the possibility of developing Multiple Sclerosis as a secondary effect of SARS-CoV-2 infection.
COVID-19 exhibits highly heterogenous respiratory symptoms ranging from cases of hypoxia associated with respiratory failure- Acute Respiratory Distress Syndrome (ARDS) to negligible symptoms or asymptomatic conditions. Common clinical symptoms of COVID-19 include fever, dry cough, and fatigue with less common symptoms, including sore throat, headache, diarrhea, loss of taste, or smell [1]. Significant clinical symptoms caused by SARS-CoV-2 in COVID-19 include pneumonia, lower respiratory symptoms like cough and shortness of breath, fever, fatigue, and in some cases with less common symptoms of headache, sputum production, diarrhea, and upper respiratory tract symptoms like coryza breath [2]. Additionally, it is important to note that cases which exhibit headaches, a loss of smell and/or taste, confusion, dizziness, and impaired consciousness highlights an essential and influential link between SARS-CoV-2 infection and the CNS. Hence its potential impact on the long-term progression or development of the CNS is necessary to understand.
Statistical reports by WHO confirmed about 89% of all COVID-19 associated deaths occurred in individuals above 65 years of age: of which 95% had at least one underlying health condition, and 57% of all deaths reported are men. Studies have further confirmed that the prevalence rate of COVID-19 on both men and women are comparable, but men are at higher risk of severe outcomes and death independent of age. Details regarding SARS-CoV-2 are explained in Figure 1.
Figure 1: Different types of coronavirus infections: its source and intermediate host - SARS-CoV-1 (SARS), MERS CoV (MERS), and SARS-CoV-2 (COVID-19). Figure details on SARS-CoV-2 that cause COVID-19. SARS-CoV-2 is a single-stranded beta coronavirus with the similar genome sequence of SARS-CoV-1 that stays in surfaces like air, copper, cardboard, stainless steel, and plastic for longer hours. SARSCoV- 2 is mainly transmitted by respiratory droplets, saliva, urine and feces.
Transfer and entry of SARS-CoV-2
The mode of zoonotic transfer of coronavirus from bats to humans in SARS, MERS, and COVID-19 is via an intermediate host like civet cats, camels, and pangolins, respectively. SARS and SARS-CoV-2 enter humans via the Angiotensin-Converting Enzyme 2 (ACE2) receptor, mainly expressed in the lungs, brain, heart, blood vessels, gut, kidney, and testis. Computational analysis has suggested that the zoonotic transfer of the SARS-CoV-2 virus would occur via a binding mechanism between ACE and TMPRSS2. ACE2 receptor allows the entry of SARS-CoV-2 to humans while the serine protease TMPRSS2 primes the spike protein (S) of SARS-CoV-2 (Figure 2). Interestingly, although SARS and SARS-CoV-2 exhibit amino acid sequence a similarity, surface plasmon resonance sensorgram determined the binding capacity of the human ACE2 receptor, and the spike protein to SARs-COV-2 is of greater affinity (about 10 to 20 fold high) than ACE2-S binding of the SARS-CoV. This increased affinity of the SARS-CoV-2 to ACE2 receptors could explain the vigorous virus transmission and severe infections of COVID-19 compared to other coronavirus infections.
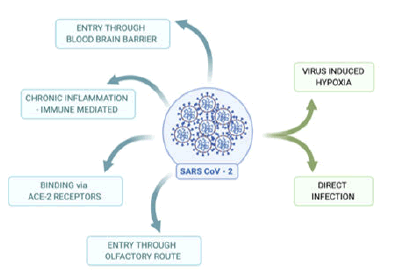
Figure 2: Possible routes of entry of SARS-CoV-2 to the brain to cause infection: Blue color: confirmed routes of entry. In humans, SARS-CoV-2 binds to ACE-2 receptors, migrates via olfactory route, and cross Blood- Brain Barrier (BBB) to enter CNS to cause brain infection. SARS-CoV-2 could also mediate via an immune-mediated pathway to enter the CNS. Green color: Route that needs further study in association with SARSCoV- 2 includes virus-induced hypoxia and direct infection to the brain.
SARS-CoV-2 - Entry and effect in the nervous system
Apart from the generic respiratory complication caused by a SARSCoV- 2 infection, a plethora of evidence supported the potential effect of SARS-CoV-2 on both the Central Nervous System (CNS) and the Peripheral Nervous System (PNS) [3]. The effects of SARS-CoV-2 infections on the CNS include headache, loss of consciousness, vertigo, acute cerebrovascular disease, loss of muscle control (ataxia), and seizure, while that on the PNS includes loss of smell, taste, vision, and episodes of neuropathic pain. A case-study conducted confirmed that many of these symptoms arise in 78 out of 214 hospitalized COVID-19 cases (36.4%). Furthermore, several other case study reports associated with SARS-CoV-2 infection also revealed that in addition to the above symptoms, patients also suffered from muscle pain, encephalitis, encephalopathy, epileptic seizures, stroke, rhabdomyolysis and Guillain- Barre syndrome. Moreover, genome sequencing confirmed the presence of SARS-CoV-2 in Cerebrospinal Fluid (CSF) of infected persons proving the SARS-CoV-2 entry and effect on the CNS.
Neuro-invasive and neurotropic SARS-CoV-2 has been implicated in exerting different stages of infection, starting with a marked loss of smell and or taste during early stages of infection to immunomodulatory effects affiliated with seizures at later stages. As neurotropic viruses enter the CNS from the primary site of infection, it invades the nervous tissues and disrupts its homeostasis causing infections. Once an organism meets a pathogenic protein like bacterial and viral proteins, membrane-bound receptors known as Pattern Recognition Receptors (PRRs) (eg: C-type lectin Receptors (CLRs) and Toll-Like Receptors (TLRs)) activates several immunological signaling pathways to remove the pathogenic protein from the organism. TLRs are mostly expressed in cells, and it recognizes most pathogenic protein, thereby activating a cascade of signaling pathways. Activation of TLRs leads to the production of inflammatory mediators like cytokine and chemokines via an interplay of immune cells like monocytes, mast cells, macrophages, and lymphocytes [4]. Inflammation of the CNS, known as neuro- inflammation, includes both immunological and neuronal cells and results in modulation of the immune response of the nervous system and the synaptic plasticity. The brain is highly guarded and protected by the Blood-Brain Barrier (BBB) and blood-CSF barriers to protect the brain from the entry of external molecules, pathogens, and cells. BBB mainly consists of astrocytes, pericytes, extracellular matrix, and cerebral micro-vascular endothelium, mainly Brain Micro-vascular Endothelium Cells (BMECs), and they maintain the permeability of the BBB via Tight Junctions (TJ) by continuous capillaries with no fenestrations [5].
The entry of SARS-CoV-2 into the CNS could occur via the hematogenous or peripheral nerve routes. The hematogenous route would be the main route for the neuro-invasiveness of SARS-CoV-2 and could be via the Trojan Horse mechanism which most of the neurotropic viruses adapt to enter the CNS. Trojan Horse mechanism refers to the ability of a pathogen to infect the CNS by crossing BBB via transcellular, paracellular and/or via infected phagocytic cells. The peripheral nerve route would be via the olfactory system/olfactory bulb [3].
Hematogenous route of viral infection
Upon infection, the neurotropic virus extends its reach into the CNS through the bloodstream via transcellular and paracellular routes that cross the BBB. Entry through a transcellular route involves the passage of the virus-mediated via transcytosis through BMECs or endocytic vesicles of pericytes. Also, SARS-CoV-2 has further been indicated to have the capacity to cross the BBB by infecting endothelial cells, through a mechanism called viremia. Viremia is the process by which the neurotropic virus-like SARS-CoV-2 enters the bloodstream after the primary infection to reach the CNS. On the other hand, viral entry through to the CNS via the paracellular route is, in a sense, a Trojan Horse mechanism. During systemic infection, viruses opt paracellular route associated with the release of cytokines, chemokine, and Matrix Metalloproteinase (MMPs), leading to an infection of leukocytes (macrophages or monocytes) in the bloodstream and BBB inflammation. This allows the transmigration of the virus to the CNS via the Para cellular route using the Trojan Horse mechanism.
2. Peripheral nerve route of viral infection
The communication between peripheral tissues and the CNS is necessary for the proper functioning of an organism, and neurons play a preeminent role in this communication. Neurons innervate the peripheral organs, and they act as the gateways for the virus to enter the CNS. Neuronal polarization allows neurons to receive, process, and transmit information signals to other associated cells, and some viruses could infect and migrate via the nerve endings of sensory or motor neurons. Motor proteins like dynein and kinesin facilitate this viral infection and viral channeling via sensory or motor neurons via retrograde and anterograde neuronal transport. Neuro-invasion via the olfactory neurons initiates at the olfactory epithelium via bipolar cells; with its axons and dendrites reaching the olfactory bulb resulting in the formation of synapses across the cells [6]. Studies in transgenic rodent models that express human ACE2 have confirmed the transfer of coronaviruses from the nasal cavity to the CNS. SARS-CoV viral antigen was detected in olfactory bulb after 60hrs and brainstem after four days, upon intranasal administration of SARS-CoV in K18-hACE2 transgenic mice [6]. A recent study by Dube et al in 2018 also confirmed the coronavirus’s entry to the CNS via the olfactory bulb. Intranasal exposure of human coronavirus- HCoV-OC43 on 15-day old C57BL/6 mice presence of a virus on piriformis cortex, spinal cord, and brain stem after four days of exposure. These in-vivo studies confirm the neuro-invasive nature of human coronavirus. The entry of the virus to the CNS alters the neurons and marks the initial step for a disease progression with its neurotropic nature and the associated immune response. Collectively, this depicts the potential and impactful neurotropic influence of SARS-CoV-2 within the nervous system.
In the CNS, as the SARS-CoV-2 virus reaches the CNS via hematogenous route and/or peripheral nerve route, immune cells that migrate to the CNS along with the resident glial cells play an intracellular and intercellular communication interfering the brain homeostasis. The name “glial cells” originates from an ancient Greek word “glía” which means “glue” in English. Glial cells constitute about 33-66% of the total brain and are mainly subdivided into four groups of cells; 1) microglia, 2) astrocytes, 3) oligodendrocytes, and 4) Nerve/glial antigen 2 (NG2)-glia of which the most widely popular glial cells are microglia, astrocytes, and oligodendrocytes. Here, we briefly discuss on glial cells and its role in CNS.
Microglia
Microglia has a vital role in the maturation of the normal brain and several other processes including angiogenesis, proliferation of astrocytes, differentiation of neurons, growth of axons, embryonic cortical precursor cell development, and apoptosis. Microglias, the CNS resident phagocytic cells, induce innate and adaptive immune responses via inducing a cytotoxic effect, the release of pro-inflammatory cytokines and chemokines, and the regulation of T lymphocyte responses via antigen presentation or by phagocytosis. In adult CNS, under normal physiological conditions, microglia exists as quiescent or resting cells constantly monitoring its surrounding microenvironment and interacting with blood vessels and astrocytes and does not exhibit any inflammatory responses. Any stimulus like infection, tissue damage/injury, or protein aggregates would activate microglia (known as the reactive microglia), thereby stimulating an inflammatory role to clear the infectious pathogen and regenerate cells that are damaged. Microglia exerts either a disease progressive or protective nature in the CNS. The disease progression role of microglia is associated with the type of injury and the activating or inhibiting microenvironment; microglia would intensify injury and lead to neurodegeneration. Alternatively, the protective role of microglia includes tissue repair and regeneration.
Astrocytes
Astrocytes form the most abundant cell type in the CNS with key roles in providing nutrient supply, recycling neurotransmitters, and maintaining homeostasis. As CNS injury/insult occurs, astrocytes initiate a defensive mechanism called reactive astrogliosis to regulate stress, tissue damage, and homeostasis. In other words, reactive astrogliosis is the process by which astrocytes maintain the CNS’s normal functioning. Astrocytes exert mainly a suppressive role in inflammatory responses and would produce several pro- or anti-inflammatory cytokines.
Oligodendrocytes
Mature oligodendrocytes produce myelin sheaths of multiple axons, which are essential for saltatory conduction of action potentials and are highly sensitive to reactive oxygen species and oxidative stress and play a role in immune-mediated functions in the CNS alongside microglia and astrocytes. The second type of fleeting premyelinating oligodendrocytes also exists in the CNS, they act as a reservoir of cells to further differentiate into mature myelinating oligodendrocytes and they are mainly present in the gray matter of the cerebral cortex (less myelinated brain regions). As oligodendrocytes are cells with high energy and metabolic demand, they depend on the microglial derived molecules and thereby release myelin of low quality leading to the neurological disease. With stress, oligodendrocytes release immune mediators and help recruit microglia to the site of the damaged tissue via the expression of chemokines like CCL2, CCL3, and CXCL10. Oligodendrocytes contain several myelin-specific enzymes that depends on calcium-like neutral proteases and protein kinases, especially granzymes (serine proteases), which could lead to hydrolysis of myelin basic protein, thereby disrupting the integrity of myelin sheaths.
Nerve/glial antigen 2 (NG2)-glia
Recently, NG2-glia cells are considered a relevant group of cells as they are present in both the white and gray matter of the brain and are well developed in the both postnatal and adult brains. Morphology and functional properties like membrane channels and receptors and interactions with other neural cells of NG2-glia cells differ in different brain regions making their distinct and important glial cells type. NG2-glia cells are the progenitor cells of mature oligodendrocytes, astrocytes and capable of forming functional synapses with neurons that function uni-directionally. Uni-direction neuronal signals imply that the NG2-glia cells could only receive neuronal signals and cannot generate action potentials.
Shreds of evidence suggest that the coronavirus infection could be associated with Multiple Sclerosis (MS). MS is a classic example of a demyelinating disease characterized as an autoimmune inflammatory disease with chronic demyelination of the white matter with unidentified etiology. Some of the clinical manifestations associated with MS include fatigue, muscle spasms, depression, cognitive dysfunction, seizure, focal sensory loss, vertigo, ataxia, and trigeminal neuralgia. Demyelination is generally referred to as the process by which the myelin sheaths around the axons are lost/removed, and occurs in both CNS and PNS. Demyelination affects memory function, and survival of neurons as demyelination in the hippocampus leads to reduced expression of neuronal genes. This, in turn, would affect axonal transport with decreased synaptic density, glutamate receptors, and reduced intermediates. In addition, demyelination would also result in the development of neurodegeneration. In MS, due to demyelination, continuing irreversible decline in neurological function occurs with progressive axonal damage due to the loss of connection between axons and myelin sheath. This leads to axons with swelling, reduced caliber, and degeneration with continual advancement in the development of MS. Demyelination is reversed by spontaneous remyelination by the oligodendrocytes, and its balance is mainly maintained by both innate and adaptive immune systems determining the effect and severity of the demyelinating disease. Neuronal damage in MS is mostly associated with excitotoxicity via glutamate, significantly increasing in the CSF and brain of MS patients. In MS, in the hippocampus, inflammatory systems are linked directly to synaptic dysfunction which includes eliminating the synapses with the activation of the complement system.
Mouse models and studies associated with epidemiological analysis and identical twins confirmed the association of MS with viral infections. Earlier studies in 1980 have shown the presence of both coronavirus like particles in patients with MS. For this, histologic analysis performed on the brain tissues collected from autopsies of MS patients observed demyelinated area surrounded with astrocytes and these brain tissues were inserted into the intracerebral area of weaning mice and later, the coronavirus-like particle was confirmed in cell-culture systems, the livers and brain of the infected suckling mice. The presence of RNA in CSF and antibodies of human coronavirus have also been detected in MS patients confirming an infection of the CNS followed by a coronavirus infection. Apart from the immediate effect of a viral infection, the virus could stay in the body in a dormant phase and a latent phase of the virus would be followed by reactivation of its viral activity and would lead to oligodendrocytes lysis to progressive multifocal leukoencephalopathy or demyelination; a condition usually associated with coronavirus infections. Hence the possibility of MS followed by SARS-CoV-2 infection as a long-term effect of these viral infections on CNS cannot be ruled out
Furthermore, the Mouse Hepatitis Virus (MHV), a type of coronavirus that infects mice, has been widely used to understand the neurological manifestations on CNS, thereby developing MS upon coronavirus infection. Administration of MHV to mice, via an intracranial route, induced acute severe encephalomyelitis in mice affecting astrocytes, microglia, and oligodendrocytes. Although, after two weeks of administration, there were no viral loads detected in animals that survived, oligodendrocytes expressed viral antigen in the survived mice. This shows the exertion of viral activity by MHV, with a progression of a demyelination disease mediated by several immune cells. MHV is considered the perfect model to study MS pathogenesis as they show both demyelination and remyelination in mice model upon MHV infection, which is a vital characteristic in MS.
Both intranasal and intravenous administration of MHV on mice and primates caused CNS infection and confirmed the neurotropic effect of coronavirus. It is often associated with the downregulation of IFN- in Brain Microvascular Endothelial Cells (BMEC), causing acute encephalomyelitis and demyelination. Demyelination due to MHV infection involves the activation of microglia and immune cells mediated inflammatory responses. This study provides a strong base to understand the MS pathology by coronavirus infection. Hence, it is likely that the patients with SARS-CoV-2 infection would develop earlier, and delayed responses of neurological complications and MS would be delayed.
The initial presentation of MS occurs between 15-55 years and is mainly reported in women than men. In the early development stage of MS, inflammatory cells induced demyelination is associated with different processes like activation of the microglia, oxidative stress, and damage of mitochondria in the axons). Amplification of this process would depend on the brain’s complexity due to aging (iron accumulation in the brain with aging). Damage of mitochondria in the axons would lead to prolonged stress in cells and loss of ionic homeostasis leading to the death of axons and neurons. A closer look into MS and animal models of MS has shown that with aging and disease progression, the remyelinating capacity of the cells deteriorates, and this leads to worsening the disease. It is also known that microglia exert its potential role in aging and neurogenesis via Fractalkine (CX3CL1; FKN). CX3CL1 signals via CX3C chemokine receptor 1 (FKN receptor) are mostly expressed in CNS neurons. The migration of microglial cells to the chemokine ligand of CX3CR1 is considered to protect the risked neurons by releasing neuroprotective and trophic factors. In stimulated microglia, signaling cascade by CX3CL1 reduces pro-inflammatory expression genes. Altered CX3CL1 signaling would induce M1 microglia phenotype activation with an increased expression of TNF- and IL-1. CX3CL1 expression is decreased with aging and leads to altered CX3CL1 signaling, which in turn leads to an overexpression of pro-inflammatory cytokines via increased M1 microglial activation. This could be a significant reason for the severity of SARS-CoV-2 infection in older adults.
In a recent case of COVID-19 confirmed cases, neurological verification has shown the presence of oligoclonal bands with the same pattern in serum and elevated levels of proteins and immunoglobulins in CSF, a reliable indicator of MS. This could be an indication of MS development in the future. However, this needs to be confirmed further if these bands were present before the SARS-CoV-2 infection. Several studies have confirmed the potential role /presence of coronavirus in MS, and hence, the potential effect of SARS-CoV-2 infection in MS development is interesting, and its possible chances and mechanism need to be further studied in research associated with SARS-CoV-2 infection.
Exposure of a genetically susceptible person to a potential/viral trigger from the environment leads to a cascade of autoimmune response leading to demyelination and MS development (Figure 3). These insults would interrupt the balance between myelin antigens in axons (as myelin sheets that surround axons) or oligodendrocytes (myelin-forming cells) and Tcells. Viral entry to the CNS could initiate the first stage of MS progression involving various types of cells, mainly innate and adaptive immune cells and glial cells. The innate immune cells respond to an external stimulus by recognizing the pathogen-associated molecular patterns (PAMPs) by the Pattern Recognition Receptors (PRRs), mainly by Toll-Like Receptors (TLR) that are cells expressed on the innate immune cells. Effector mechanisms by the activated innate immune system would include the production of nitric oxide and oxidative burst, phagocytosis of nearby pathogens, apoptotic cells, and myelin sheaths, production of chemokines and cytokines, antigen presentation to the adaptive immune cells, tropic factors secretion and release of MMPs that disturbs the extracellular matrix and the BBB. Signals from the innate immune system activate the adaptive immune system to expand the adaptive immune cells-the T-cells and B-cells.
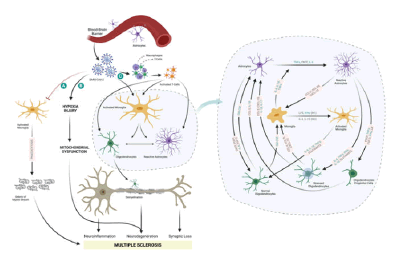
Figure 3: Possible ways by which SARS-CoV-2 leads to Multiple Sclerosis (MS).
Lesions of MS are associated with several demyelinated plaques within the white matter accompanied by a cluster of several inflammatory cells like activated microglia, lymphocytes, and macrophages. Inflammatory and neurotoxic responses in MS lesions by reactive astrocytes cause damage to the tissues via manipulating glutamate (increased) and redox homeostasis. However, astrocytes are considered to play a central role in dampening the inflammation, thereby promoting neuroprotection and repairing lesions in MS. Scattered plagues in MS formed due to demyelination is enclosed with reactive astrocytes and might exert emperipolesis, where the astrocyte engulf one or more cells like oligodendrocytes or lymphocytes. However, the role of emperipolesis in MS is yet not clear. Demyelination is also associated with cytotoxic T cells (CD8+ T cells), which releases perforin-pore forming cytolytic protein that has defined roles in suppressing and inactivating CD4+ T cells. Perforin promotes astrocyte activation, disrupts tight junction organization, and increases vascular permeability of CNS. Perforin induces apoptosis in oligodendrocytes leading to repair of myelin sheath in the CNS. Calcium ions could mediate this. In MS, oligodendrocytes are reduced in numbers and show signs of stress and apoptosis, swelling with complement deposition, and cell lysis.
Neurological complications of SARS-CoV-2 infection are found to be associated with encephalitis, encephalopathy, and Acute Disseminated Encephalomyelitis (ADEM). Encephalitis, inflammation of the brain, is caused due to direct infection by viruses known as acute encephalitis or due to an immune response corresponding to an infection known as postinfectious encephalitis or ADEM. Acute encephalitis appears within days or periods of one or two weeks and would interfere with the consciousness of the patient and show symptoms of headache, lack of orientation, and neurological issues. Later this would show inflammation of CSF around the brain and be associated with fever, behavioral changes, abnormal brain waves/ electroencephalogram, and variations in computed tomography scan or brain MRI.
Several studies have concluded SARS-CoV-2 associated encephalitis, with a potential effect of viral infection on the CNS of these patients. A 54-year-old woman confirmed to have SARS-CoV-2 was found unconscious, for which the doctors concluded the neurological effect of SARS-CoV-2 infection. The patient showed anosmia and dysgeusia at an early stage of the disease with seizures at the later stage of the disease with severe hypoxia. Seizures were concluded to be an after-effect of SARS-CoV-2 encephalitis. Also, cases have been reported with encephalopathy; antibodies against SARS-CoV-2 were detected in CSF obtained from a middle-aged man and women with SARS-CoV-2. Encephalopathy is a reversible brain dysfunction caused by metabolic disorders, systemic toxemia, or hypoxia during the acute infection generally characterized by cerebral edema. Patients with infectious toxic encephalopathy display headaches, mental disorders, disorientation, paralysis, loss of consciousness, and coma. Interestingly, COVID -19 patients have also reported viremia, severe hypoxia and lately associated encephalopathy, however more studies are still needed to confer the pathophysiology behind the development of viral encephalitis or encephalopathy associated with SARS-CoV-2 infection in COVID-19.
Acute Disseminated Encephalomyelitis (ADEM) is a rare demyelinating disease of the CNS which progresses rapidly with autoimmune processes followed by an infection via viral exposures or immunization. ADEM is associated with fever, meningitis, seizures, and unconsciousness. It is generally seen in children than adults, with a slight predominance in females than males [8]. ADEM could also be confirmed by MRI, CSF analysis for lymphocytes, and myelin basic protein. ADEM is mostly monophasic, with rare cases of relapsing ADEM, making it a diagnostic challenge to distinguish from MS. Clinically, MS and ADEM represent demyelinating autoimmune diseases that affect the CNS. In a case study, it was observed that over three years, 35% of adult patients initially diagnosed with ADEM developed MS. Clinically, MRI confirms ADEM with T2-weighted, Fluid-Attenuated Inversion Recovery (FLAIR) hyperintensity at the grave white matter and grey-white matter interface and needs to performed in intervals of time to 2-3 years to confirm a diagnosis of ADEM as with time, accumulation of T2 lesions could occur, and these patients would need to be re-considered for MS development.
Interestingly, several case reports of COVID-19 infected patients with a possible association with ADEM have been published. A 51-year-old women with a confirmed case of COVID-19 with persistent fever and no clinical advancement and no neurological history was concluded to have ADEM. Brain MRI images showed hyperintense lesions on FLAIR imaging in the grave white matter, while the CSF analysis showed no signs of direct infection of the CNS. Another study has also confirmed ADEM in a 64-year-old woman as a case of an immunemediated effect on CNS that occurred after SARS-CoV-2 infection. These studies confer a strong association of SARS-CoV-2 infection with ADEM, and this could be considered an early symptom of similar patients to develop MS in the future via a direct or indirect effect of the virus. Moreover, studies have confirmed the development of pediatric MS in children within years after the occurrence of ADEM and hence with our current understanding on SARS-CoV-2 infection, in addition to the development of encephalitis/ encephalopathy and ADEM as an immediate neurological complication, within a decade or two, the secondary effect of SARS-CoV-2 infection could be marked with an increased number of MS patients.
As mentioned earlier, based on our knowledge of SARS-CoV-2 infection and the presence/ability of coronavirus to develop MS in patients and in vivo models, respectively, a future wave of MS could be strongly predicted as similar to Parkinson’s disease followed by the influenza pandemic of 1918 (The Spanish Flu). Encephalitis/ Encephalopathy and ADEM could be considered the first line of neurological effect with a later episode of MS development. In this section, we discuss the possible mechanism that could mediate MS development followed by SARSCoV- 2 infection.
Cytokine storm and neuroinflammation
Clinical data confirm an association of an immunological effect of SARSCoV- 2 infection leading to a cytokine storm. A cytokine storm is characterized as a critical immune response with prompt hyperactivation and proliferation of immune cells like natural killer cells, macrophages, and T-cells with glial cell activation in the CNS causing neuroinflammation and demyelination. In viral infections, this cytokine storm would lead to the apoptosis of the lungs’ epithelial and endothelial cells and would consequently result in vascular leakage, hypoxia, and alveolar edema. Upon a pathogenic insult to the organism, virus itself or the cytokines could cross BBB via BBB transporters and circumventricular organs and activate glial cells (microglia, astrocytes, and oligodendrocytes), mostly microglia initiating an intricate neuroinflammatory signaling cascade with the release of several cytokine and chemokines. Neuroinflammation is associated with the release of numerous pro-inflammatory factors like TNFα, IL-1β and nitric oxide free radicals leading to the subsequent recruitment of more macrophages and microglia to the CNS to remove the cell debris produced during the neural injury. This continual exposure of neurons to pro-inflammatory cytokines would result in neuronal dysfunction and degeneration that are mainly associated with the development of age-related neurodegenerative diseases. Activation of astrocytes by pro-inflammatory cytokines, stress (oxidative or chemical), pathogen-associated molecular patterns (PAMPs) leads to the expression or upregulation of cytokines (TNF-α, IL-6 and IL-1β), chemokines (CCL2, CCL20, and CXCL10), neurotrophic factors including Nerve Growth Factor (NGF), Brain-Derived Neurotrophic Factor (BDNF), Vascular Endothelial Growth Factor (VEGF), and Leukemia Inhibitory Factor (LIF), MHC II cell adhesion molecules such as ICAM-1, VCAM-1 and TLRs. These molecules play a crucial role in killing the invading pathogens; however, they would also exert bystander damage to the adjacent glia cells and neurons.
Cytokine profiling of COVID-19 samples mainly with patients admitted in the intensive care unit have shown an increase in IL-2, IL-7, GM-CSF, IFN- γ inducible protein 10 (IP-10; CXCL10), MCP-1, MIP-1, and TNF- α which could cause viral-induced hyper-inflammation [9]. Also, severe cases of COVID-19 cases have shown an increase in IL-1β, IL-1ra, IL-2R, IL-6, IL-8 (CXCL8), IL-17, IFN-γ, and GM-CSF (Figure 3/ Table 1).
Table 1. List of cytokines/chemokines associated with the cytokine storm in SARS-CoV-2 infection association with Multiple Sclerosis (MS).
| Name | General Multiple Sclerosis (MS) Associated Function |
|---|---|
| Interleukin-2 (IL-2) | In MS, plays a role in the loss of immune tolerance. |
| In MS, helps in the proliferation of autoreactive T cells. | |
| Interleukin-6 (IL-6) | T cell expansion, proinflammatory |
| Interleukin-17(IL-17 | Reduced lesion activity, demyelination in MS |
| Interleukin-10 (IL-10) | Anti-inflammatory, Decreased antigen porfesentation monocytes and macrophages; Neuroprotective |
| In MS, IL-10 is decreased prior to relapse and increased during remission | |
| Interleukin-7 (IL-7) | Lymphocyte development, Increased risk of MS |
| Interleukin-8 (IL-8 )/ Chemokine (C-X-C Motif) Ligand (CXCL8) | Chemo-attractant for neutrophils and monocytes, |
| In MS, monocyte recruitment to the CNS | |
| Interleukin-1 (IL-1) | Proinflammatory, pathogenic role in MS |
| Granulocyte- Macrophage Colony Stimulating Factor (GM-CSF) | Regulation of microglial functions, stimulation of microglial priming for antigen presentation, pathogenic action in MS |
| Interferon gamma (IFN- gamma) | Drives inflammation |
| Tumor Necrosis Factor alpha (TNF-α) | Proinflammatory |
| Transforming Growth Factor beta (TGF-b) | Lymphocyte proliferation, differentiation, and survival, protective effect in MS |
| Interferon-gamma- inducible Protein-10 (IP-10/CXCL10) | Pathogenesis in MS |
| Nitric Oxide (NO) | In MS Dual role- immunomodulatory, Disrupts BBB, demyelination, axonal degeneration |
| Monocyte Chemotactic Protein-1 (MCP-1) | Pathogenesis in MS |
In the CNS, the infiltration of various immune cells and cytokines released from these cells leads to inflammation of white and gray matter (neuroinflammation), leading to MS development. This includes the association between the myelin-specific T helper (Th) cells and Major Histocompatibility Complex (MHC) class II presenting alleles Antigen- Presenting Cells (APCs). The ligand-binding receptor of the Pathogen- Associated Molecular Pattern molecules (PAMPs) in viruses or bacteria, binds to Toll-Like Receptors (TLRs) expressed on the surface of TLRs leading to the release of various cytokines like IL-4, IL-12, and IL-23. In the presence of these cytokines, CD4+ T cells differentiate into Th1 (proinflammatory), Th2 (anti-inflammatory), or Th17 (pro-inflammatory) phenotypes releasing specific cytokines. Proinflammatory cytokines like TNF-α and IFN-γ are released by Th1 cells which suppress the differentiation of Th2 cells. The anti-inflammatory role of Th2 cells is exerted by IL-4 and IL-13, of which IL-4 decreases inflammation via activation and increase in M1 and M2 (repair) macrophages. The antiinflammatory role of IL-13 is exerted via releasing MMPs. The IL-17, IL-21, IL-22, and IL-26 mediate inflammatory response of Th17 cells in MS.
Within the CNS, neurotropic and neurotoxic SARS CoV-2 would interfere with demyelination/remyelination, neurodegeneration, neuroinflammation and synaptic loss of neurons leading to MS progression. Possible ways: A) Reduced phagocytosis of myelin sheath debris, SARS-CoV-2 might decrease the phagocytic capacity of microglia cells, and macrophages of myelin sheath debris; accumulation of myelin sheath debris hinder the access of the remyelinating cells like Schwann cells causing MS; B) Hypoxia-induced Mitochondrial dysfunction; C) Cytokine storm and increased demyelination- entry of SARS-CoV-2 could activate immune cells (macrophages and T-cells) and glial cells, with increased expression of several cytokines, interleukins and chemokines thereby leading to demyelination. Blue coded cytokines, interleukins, and chemokines represent the molecules involved in SARSCoV- 2 infection.
Altering the phagocytotic capability of microglia/macrophage
SARS-CoV-2 could alter the demyelination/ remyelination equilibrium by microglia and macrophages in the brain, and this could result in the accumulation of myelin sheath debris and MS development. Both microglia and macrophages are of myeloid orign and play a crucial role in phagocytosis. As the resident macrophage cells in the CNS, microglia mostly have a role in removing cell debris after ischemia or damage to the myelin sheaths and is a crucial process for efficient remyelination followed by demyelination of axons. Microglial phagocytosis occurs during neuronal connections restructuring, acute CNS injury, MS, and aging via three main mechanisms:
a) Via phagocytosis of myelin and extracellular aggregates like amyloid.selcitrap.
b) Via release of growth factor, neurotrophic factors, and antiinflammatory cytokines would stimulate axon branching and repair myelin sheaths.
c) Via recruitment of stem cells and other precursor cells and triggering astrocytes to release trophic factors that would neurons to develop and maintain synaptic connections.
Accumulation of myelin sheath debris would lead to the formation of a dense matrix surrounding demyelinated axons thereby blocking the remyelinating cells to demyelination sites. Hence, efficient removal of myelin sheath debris would ease the access of remyelinating cells to the demyelinated axons. This accumulation of myelin sheath debris could also affect remyelination by blocking maturation of the oligodendrocyte progenitor cells. Studies in the demyelination model by Kotter et al observed impaired remyelination with a decrease in macrophages and microglia with decreased removal of myelin sheaths debris. Hence, a possible mechanism for MS development followed by SARS-CoV-2 infection could be via accumulation of myelin sheath debris due to fewer microglia/macrophages in CNS. Thus SARS-CoV-2 could increase stride of demyelination of axons in CNS leading to MS progression by interrupting phagocytotic role of macrophages/ microglia and thereby hindering remyelination.
Hypoxia mediated mitochondrial dysfunction and neurodegeneration
Recent findings on the mitochondrial involvement in MS pathogenesis are interesting and this correlates with another probable secondary effect by SARS-CoV-2 infection. As SARS-CoV-2 infection-associated encephalopathy is related to hypoxia, hypoxia-induced mitochondrial dysfunction could be a possible mechanism for the progression of MS in these patients. Mitochondria play a vital role in the regulation of calcium and ATP synthesis and constitute a significant source of Reactive Oxygen Species (ROS). Mitochondria have a key role in maintaining the bioenergetics of a cellular environment via KREBS’s Cycle and Oxidative phosphorylation, cell-signaling, calcium storage, and apoptosis. In the CNS, the mitochondrial metabolic activity would also be associated with an impaired Krebs cycle or neuronal oxidative phosphorylation. Mitochondrial dysfunction leads to intracellular dysregulation and lesser energy production resulting in neuronal damage, which is highly dependent on ATP for electric signals transmission and interrupts the anterograde and retrograde transportation across the axons [10]. Therefore, as mitochondrial dysfunction is involved in MS development there is a possibility that SARS-CoV-2 infection could lead to mitochondrial dysfunction and further accelerate progression to MS development. However, this needs to be further investigated [11,12].
Currently, the world is in a race against COVID-19, with significant focus dedicated to patient care and substantial research in the development of a potential vaccine and medicine. So far, the only effective way to control the virus spread is social distancing. However, clinical data uncover the budding effects of SARS-CoV-2 within the CNS, and its potentially serious consequences in future years. The immediate neurological complications associated with SARS-CoV-2 include encephalitis, encephalopathy, and ADEM. It is essential to be cautious around the secondary effects of coronavirus exposures, as damage to the nervous system via different mechanisms is a likely risk. Moreover, one should not ignore the consideration of prospective negative impacts as the coronavirus could achieve a latent growth phase and later recur to prompt different diseases, like MS. In this review, we draw insight into the possible mechanism that would be associated with MS development in SARS-CoV-2 infected people. We focus on how the cytokines and chemokines that are known to be modulated in SARS-CoV-2 infection would interfere in the interplay of the glial cells in MS development. We further highlight the necessity of considering hypoxia-mediated mitochondrial dysfunction and alteration in the phagocytic capacity of microglia/ macrophages in the development of MS.
Future Perspectives
As there is a potential risk of MS pathogenesis as a secondary effect of SARS-CoV-2 infection, future MS development cannot be ruled out; hence, a constant and continuous follow up of exposed patients would be hugely beneficial. Moreover, this could help better understand and identify factors that may contribute towards the course of disease development, during early stages of MS, and its staged progression. Consequently, this would potentially provide further insight into effective treatment strategies and intervention, and reduce the risk of developing MS or its progression. Furthermore, studies could focus more on the hypoxia and phagocytotic role of microglia during a SARS-CoV-2 infection. Unwinding MS pathophysiology with respect to the lurking coronavirus could potentially help in an early detection of MS in SARS-CoV-2 infected individuals and could result in better medical care.
Author Contributions
NJS wrote the manuscript and designed the figures. SSA and SAA critically reviewed the manuscript.
Acknowledgments
The authors acknowledge Faizal Sherif for figure drawings.
Competing interests
The authors declare no competing interests.
Citation: Abdulla SA, et al. "Neuropathogenesis and Neurological Effects of SARS-Cov-2 on Glial Cells and Its Potential Progression to Multiple Sclerosis". J Mult Scler, 2022, 9(7),452.
Received: 11-Mar-2025, Manuscript No. jmso-20-19862; Editor assigned: 15-Mar-2025, Pre QC No. jmso-20-19862(PQ); Reviewed: 27-Mar-2025, QC No. jmso-20-19862(Q); Revised: 31-Mar-2025, Manuscript No. jmso-20-19862(R); Published: 05-Apr-2025, DOI: 10.35248/2376-0389.24.11.4.452
Copyright: 2022 Abdulla SA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.