Case Report - (2021) Volume 7, Issue 10
Epithelioid hemangioendothelioma is a rare vascular tumor. Most commonly it affects lung, liver and bones, although it may also involve the head and neck region, breast, lymph nodes, brain, skin and many other sites. Because of its heterogeneous presentation, as it represents less than 1% of all the vascular tumors, it is often misdiagnosed and not treated correctly since there are no standard treatment protocols, leading to a poor prognosis in some cases. Over 50-76% of the patients are asymptomatic. Imaging is necessary to know the involvement of surrounding tissues. It is important to recognize the expression of vascular markers (Fli-1 and CD31 are endothelial-specific markers) and the microscopic evidence of vascular differentiation to make a correct diagnosis. We focused on Radiation treatment as a good therapeutic option despite the poor prognosis, radiotherapy offers local pain control with good tolerance and better quality of life at least at a one-year followup in most of cases. Further studies are needed to establish the standard radiation dose to be used for better locoregional control of such a complex and extremely rare disease.
We report a case of a woman who found to have multiple bone and hepatic masses on imaging, initially misdiagnosed as a metastatic breast carcinoma presented with breast and axillary mass with subcutaneous nodules on forearm. The diagnosis of Epithelioid hemangioendothelioma was made on histopathological study and confirmed by immunohistochemistry, which showed diffuse response for CD31,CD34 markers and no response to tissue CEA, HMB-45 or S-100 protein.
Epithelioid • Hemangioendothelioma • Metastasis • Histopathology • Immunohistochemistry
Epithelioid hemangioendothelioma is a very rare, with only one in million people diagnosed with this cancer worldwide this grows from the cells that make up the blood vessels i.e., an uncommon low-grade malignant tumor of vascular origin that may develop in the soft tissue, lung, bone, brain, liver, and small intestine described for the first time in 1975 by Dail and Liebow. The etiology is still unknown. It usually happens in people between 30 and 50 years of age but can also occur in young children and older people [1]. In the literature, approximately 200 cases have been reported. The diagnosis can be made both on cytology as well as on histology [1,2]. We report a case of Epithelioid Hemangioendothelioma diagnosed on histopathological study, confirmed by immunohistochemistry, which shows diffuse response for CD31, CD34 marker. The recent World Health Organization classification describes Epithelioid hemangioendothelioma as lesions that fall into the category of locally aggressive tumors with metastatic potential [3,4].
A 35-year-old female presented with a four months history of hardness of right breast, mass in the Axilla, abdominal pain and multiple swelling on the right forearm with no comorbidities. Physical examination showed abdominal tenderness on the right side of the abdomen, lump in the upper outer quadrant of the right breast and axilla approximately 3*2 cms with hardness and small soft nodular hard masses three in number largest 2*1 cms in the forearm . Systemic examination was unremarkable. Routine hematological and biochemical investigations, including serum bilirubin, transaminases, alkaline phosphatase and proteins were within reference ranges. Serological tests for HIV, hepatitis B and C were negative.
25/5/21, CT scan was done showed large well defined lobulated minimal enhancing mass lesion measuring 6.5*6 cm in the right axillary region, no obvious invasion into the chest wall, adjacent axillary fat stranding noted (Figure 1).
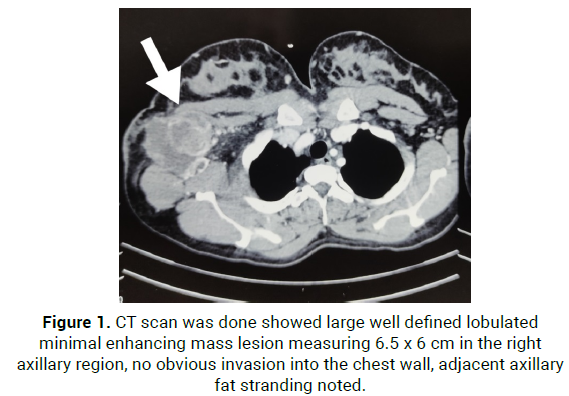
Figure 1: CT scan was done showed large well defined lobulated minimal enhancing mass lesion measuring 6.5 x 6 cm in the right axillary region, no obvious invasion into the chest wall, adjacent axillary fat stranding noted.
On 8/6/21, She was operated with wide local excision of upper outer quadrant of right breast with axillary nodal mass with overlying skin, HPR showed High grade tumor with differential diagnosis Invasive ductal carcinoma (NOS) of axillary tail of the breast mass and Non Hodgkins Lymphoma (Diffuse large B cell lymphoma) of the Axillary lymph node [recommended immunohistochemistry] (Figure 2).
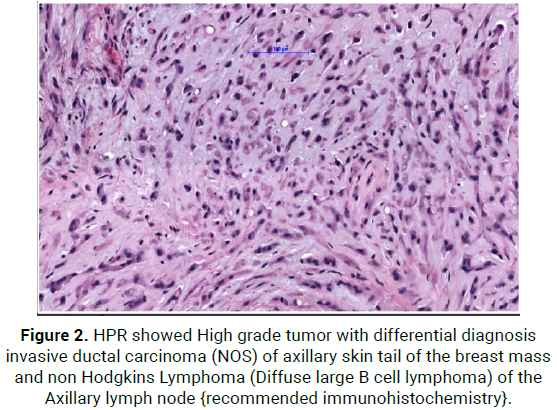
Figure 2: HPR showed High grade tumor with differential diagnosis invasive ductal carcinoma (NOS) of axillary skin tail of the breast mass and non Hodgkins Lymphoma (Diffuse large B cell lymphoma) of the Axillary lymph node {recommended immunohistochemistry}.
On 4/7/21, Excision biopsy of the subcutaneous nodule in the forearm– Malignant skin adnexal tumour, Margin <1 mm recommended again for immunohistochemistry.
On 9/7/21, Breast tumour tissue for Immunohistochemistry- Epitheliod Ha emangioendothelioma,Epitheloid mesenchymal tumour possibly vascular origin(Figure 3).
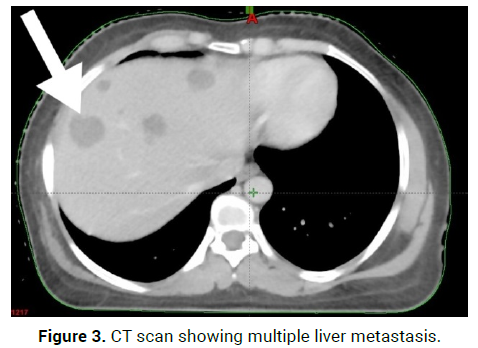
Figure 3: CT scan showing multiple liver metastasis.
Immunoreactive to vimentin, EMA, KI-67,CD1, INI-1
Ultrasound neck- normal and Chest X ray – normal
On 23/8/21-CT scan Right axillary upper outer quadrant: 2*3.5*1.8 cm enhancing lesion, Right axillary skin and subcutaneous: 2.9*3.2*1.5 cm enhancing lesion, with multiple liver metastasis and D7 and D11 vertebral Metastasis (Figures 4 and 5).
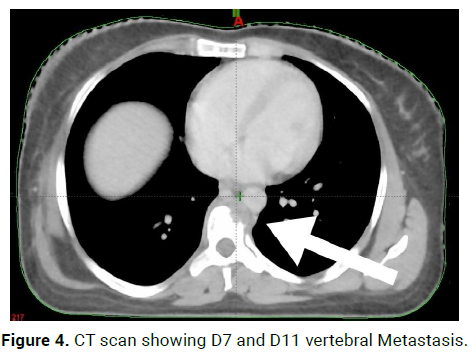
Figure 4: CT scan showing D7 and D11 vertebral Metastasis.
Figure 5: Operated with wide local excision of upper outer quadrant of right breast with axillary nodal mass with overlying skin.
We started on palliative radiotherapy 30Gy/10# to painful bone metastasis D7 and D11, planning further management with chemotherapy.
The etiology of Epithelioid hemangioendothelioma is still unknown. Many molecular theory have been implicated, different angiogenic stimulators may act as promoters of endothelial cell proliferation [5]. Recently it has been reported that monocyte chemo-attractant protein-1 is required for Epithelioid hemangioendothelioma proliferation and might promote the development of these lesions by stimulating the angiogenic behavior of endothelial cells [6]. Budousquie et al. described several clonal abnormalities in tumor cells: a complex unbalanced translocation between chromosomes 7 and 22 with multiple breakpoints, a robertsonian translocation of chromosome 14, and the loss of chromosome Y [7]. Errani, et al. focused on the t (1;3) (p36.3;q25) mutation: A molecular analysis revealed that CAMTA1 on chromosome 1p36.23 and WWTR1 on chromosome 3q25 are the involved genes.
Both genes had previously shown to play important roles in oncogenesis in Epithelioid hemangioendothelioma. Recently, Antonescu et al. have found YAP1 rearrangements in most of the transcription factor E3 (TFE3)- rearranged Epithelioid Hemangioendotheliomas, and this YAP1-TFE3 fusion subset occurs at the mean age of 30 years showing at least focally, wellformed vascular features and a variably solid architecture [8].
A new suggestive hypothesis of the pathogenesis of this disease refers to a causal relationship between chronic Bartonella infection and the development of this rare vascular tumor. The unique Bartonella’s capability of invading and inducing long-lasting intraerythrocytic and intraendothelial infections together with the ability of at least three Bartonella spp. (B. henselae, B.quintana, B.bacilliformis) of inducing vascular endothelial growth factormediated vasoproliferation, as they up regulate mitogen and proinflammatory genes resulting in cytoskeletal rearrangement and suppression of endothelial cell apoptosis, suggest that these bacterial pathogens might contribute to the development of vascular tumors [9].
Occurrence and metastasis of Epithelioid Hemangioendothelioma: The Epithelioid Hemangioendothelioma and Related vascular Disorders (HEARD) Support Group observed that the most common presentations are liver alone (21%), liver plus lung (18%), lung alone (12%) and bone alone (14%) [10]. However, it is a very heterogeneous tumor, and it has been reported to involve many other sites. When these cases occur, it may be difficult to determine if the tumor is multicentric or if it is a primary lesion with metastases in other tissues, as it may originate in either organ and metastasize to the other or it may have more than one primary site. Sometimes we are able to distinguish metastases from a multicentric primary tumor because of their less differentiation and loss of the expression of epithelial markers [11]. Distant hematogenous metastases have been reported mainly in the liver but also in the skin, serosa, spleen, tonsils, retroperitoneum and kidney; colonic metastases have also been described, but they are very rare [12]. Bone metastases are the most frequent and the most clinically severe. According to World Health Organization (WHO) classification of Tumors of bone and soft tissues (2002), one half to two thirds of them originates from a vessel small vein) [13]. Hemolytic anemia and consumption coagulopathy have been rarely described.
Laboratory data of Epithelioid Hemangioendothelioma: Blood counts are usually normal. There is no effect on the total white blood cell count, erythrocyte sedimentation rate or serum proteins, but several values might abnormally increase if the liver is involved: white blood cell count, serum alkaline phosphatase, aspartate aminotransferase, gammaglutamyl transferase, amylase and lipase. Chemistry profiles and urinalyses are generally normal [14].
Radiological findings: The imaging evaluation of the Epithelioid Hemangioendothelioma must be as complete as possible. The radiology examination and magnetic resonance imaging are necessary to obtain morphological data: the degree of the neoformation, the relations with the surrounding tissues [15].
In Pulmonary Epithelioid Hemangioendothelioma radiologically appears as multiple perivascular nodules with well-defined or blurred margins in both lungs, showing little or no growth on serial chest radiographs or computed tomography. The nodules may range in size up to 2cm, but most are less than 1cm in diameter and are predominantly in the lower parts of the lungs. They are usually found at small and medium sized vessels and bronchi. Bilateral multiple nodular opacities are the most common presentation in about 60% of cases occasionally develops as a solitary, lung nodule, measuring up to 5 cm (10-19% of cases13). Hilar lymph node enlargement, lymph node metastases, interlobular septal thickening and pleural effusions can be also noticed, as well as unilateral opacities and mosaic areas of ground-glass attenuations. Radiologic calcification is not common but histologic examinations often reveal calcified and ossified necrotic centers of the nodules [16].
Bone metastases of Epithelioid Hemangioendothelioma involve more than 50% of the cortex, there is a serious risk for pathological fractures when they arise in vertebrae they might cause spine compression and therefore paraesthesia, loss of muscular strength and paraplegia [11,15]. Bone metastases appear as osteolytic lesions with homogeneous contrast enhancement on X-ray and CT scan, without matrix mineralization; osseous expansile remodeling may be seen, and joint invasion is also a common feature. These lesions can be localized in the cortical or medullary bone: cortical disruption and extension into the soft tissue can be present, so soft tissue swelling might be radiologically observed in this case. There is no specific pattern of signal intensity at MRI imaging. Most frequently Epithelioid Hemangioendothelioma shows low to intermediate signal intensity on T1-weighted images and high signal intensity on T2-weighted images, together with homogeneous enhancement after the injection of contrast medium [17]. In some cases, positron emission tomography17 and bone scintigraphy are used to evaluate lesions at other sites. Increased uptake of F-fluorodeoxyglucose in this tumor has been recently reported [18].
Histological and cytological features: Epithelioid Hemangioendothelioma identified in electron microscopy shows typical image of endothelial cells similar to those composing medium-size vessels, or a large vein, arranging in nests or cords. The cell growth may form lumens of various size which occasionally contain red blood cells, that is the numerous distinct cytoplasmic vacuoles which may be observed under a microscope [19]. Some smears have been reported to contain cells arranged as single cells and pseudopapillary and pseudoglandular structures of varying size, or complex branching cell groups with central stromal cores, as well as cell aggregates lacking sharp anatomic borders or scalloped outlines.
Immunohistochemistry: A variety of endothelial proteins may be useful to identify Epithelioid Hemangioendothelioma. The Fli-1 protein is expressed by the endothelium as well as the T-cells and megakaryocytes this nuclear protein has proven useful in identifying vascular neoplasm including Epithelioid Hemangioendothelioma, showing a better combined sensitivity and specificity than the endothelial markers CD31 and CD34. CD34 is reported to be expressed by more than 90% of vascular tumors, so this marker has poor specificity as a variety of soft tissue tumors also express it. In contrast, CD31 is regarded as a relatively specific vascular tumor marker. Some authors maintain that the combination of Fli-1 and CD31 represents an ideal panel for the differential diagnosis.74 Podoplanin is specifically expressed by lymphatic endothelial cells and has been reported to be a useful diagnostic marker to identify Epithelioid Hemangioendothelioma in the liver.
Prognosis and death causes: The mean survival is 4.6 years, ranging from 6 months to 24 years [20]. Gomez-Arellano et al. reported that mortality is 13% when Epithelioid Hemangioendothelioma is located in soft tissue, 35% when it affects the liver and 65% if it reaches the lung. Patients with hilar metastases or liver involvement reported to have a worse prognosis, with an average survival of 2.2 years [21]. Epithelioid Hemangioendothelioma progresses on average in 2 years, but the presence of metastasis at the time of diagnosis does not necessarily correspond with reduced survival. Histological finding as spindle tumor cells, fibrinous pleuritic lesions or extrapleural tumor cells proliferation show a trend towards a worse prognosis. Most patients die from respiratory failure as a result of tumor nodules increasing in size and number and those with hemorrhagic effusion, a rapid respiratory failure is usually the cause of death [22]. A small group succumbs because of extrapulmonary spread of tumor, with a predominance of liver and bone disease [23].
Treatment options
Because of its rarity Epithelioid Hemangioendothelioma has no standard for treatment, and actually few therapeutic options are available. If the proposed association of Bartonella spp. infections were confirmed, it would be plausible that eradicating the bacterial infection or interrupting Bartonella-induced angiogenic and proliferative cell signals could slow the tumor progression and improve patient outcomes.
When the lesions are small and limited in number, some authors recommend surgical resection or an preventive approach to asymptomatic patients. Successful curative resection achieves good outcomes. The role of adjuvant chemotherapy and/or radiation therapy (RT) is ambiguous, instead. Usually RT after surgical resection is chosen for localized Epithelioid Hemangioendothelioma, in order to control the residual disease given the recurrence of Epithelioid Hemangioendothelioma, while chemotherapy is preferred in case of systemic disease [24].
Lung: In patients with unilateral Epithelioid Hemangioendothelioma nodules, wedge resection offers the same survival outcomes as anatomic resection does. Hilar lymph node resection should be systematically proposed. In patients with bilateral nodules, partial or complete response has been reported using interferon-2. Corticosteroids, azathioprine, multiple wedge resections are the other proposed treatment options. Multiple chemotherapeutic regimens have been tried, but no improvement has been reported. Given its vascular origin, angiogenesis inhibition may be a reasonable approach for the management of metastatic Epithelioid Hemangioendothelioma using bevacizumab and nanoparticle albumin-bound paclitaxel [25].
Liver: Locally advanced hepatic Epithelioid Hemangioendothelioma seems to benefit from transplantation, with good results.
Bone: Radical surgery is performed for resectable bone tumors, followed by joint reconstruction. Enbloc resection is preferred in case of solitary lesions; amputation is necessary for multicentre lesions. When a pathological fracture occurs, temporary internal stabilization with a plate and screws for pain control is chosen [26].
Radiation therapy: A dose 6000 cGy adjuvant RT was performed against an axillary form of Epithelioid Hemangioendothelioma , showed a good tolerance of the treatment, neither regional nor distant metastases or local recurrence at 6, 12 and 24 month-follow-up [15].
In one study a dose of 33 fractions of RT, to a dose of 5940 cGy, was recently applied to residual, postoperative mastoid: eight years after surgery and adjuvant RT, imaging has demonstrated neither recurrence nor changes in patient’s clinical exam [27]. RT may be used above all in an attempt to sclerose the blood vessels. Further studies are needed to answer the question. Evidence is all in favor of Radiation obtaining local pain control with good tolerance and better quality of life at least at one-year-follow up.
Malignant epithelioid hemangioendothelioma is a rare tumor of vascular origin which usually occurs in soft tissues taking into account of the extremely variable nature and occurrence and its radiobiological characteristics, Radiation Therapy seems to carve out a significant role in the treatment of such a complex and extremely rare disease.
The authors acknowledge fellow colleagues for assisting and modifying contents.
The authors reported no conflicts of interest and no funding was received for this work.
Citation: Raghavendra D Sagar. Malignant Epithelioid Hemangioendothelioma: A Rare Case Report. Oncol Cancer Case Rep. 2021, 07 (10), 001-004.
Received: 30-Sep-2021 Published: 21-Oct-2021
Copyright: © 2021 Sagar RD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.