Research Article - (2023) Volume 15, Issue 4
Epilepsy is a chronic neurological disorder that manifests as a tendency to experience recurrent seizures. The management of epilepsy often involves the use of Antiepileptic Drugs (AEDs) to control seizure activity. Gabapentin, a commonly prescribed AED, has shown efficacy in the treatment of epilepsy. This abstract aims to summarize the effectiveness of gabapentin in managing epilepsy based on available clinical evidence.
Numerous studies have investigated the efficacy of gabapentin in epilepsy treatment. Clinical trials and retrospective studies have consistently demonstrated the beneficial effects of gabapentin in reducing seizure frequency and improving overall seizure control. Gabapentin's mechanism of action involves binding to the α2δ subunit of voltage gated calcium channels, modulating neurotransmitter release and dampening excessive neuronal excitability.
Furthermore, gabapentin exhibits a favorable safety profile, with most adverse effects being mild to moderate in nature. Common side effects include dizziness, somnolence, and mild gastrointestinal disturbances. The incidence of serious adverse events is relatively low, making gabapentin well-tolerated by patients with epilepsy.
Nature • Epilepsy • Patients • Dizziness • Drugs
Epilepsy is a chronic neurological disorder characterized by recurrent and unpredictable seizures, affecting approximately 70 million people worldwide. The management of epilepsy typically involves the use of Antiepileptic Drugs (AEDs) to control seizure activity and improve the quality of life for individuals living with the condition. Among the wide range of AEDs available, gabapentin has emerged as a valuable treatment option for epilepsy [1].
Gabapentin, chemically known as 1-(aminomethyl) cyclohexaneacetic acid, was initially developed as an anticonvulsant and approved by the United States Food and Drug Administration (FDA) in 1993 for the treatment of partial seizures in adults. It belongs to the class of structural analogs of γ-aminobutyric acid (GABA) and primarily acts by binding to the α2δ subunit of voltage gated calcium channels (Figures 1 and 2). This interaction reduces calcium influx, modulates neurotransmitter release, and ultimately suppresses excessive neuronal excitability, leading to the control of seizure activity [2].
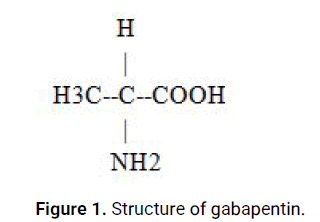
Figure 1: Structure of gabapentin.
Figure 2: Gabapentin oral tablets.
Numerous clinical studies have investigated the efficacy of gabapentin in epilepsy treatment. In a randomized, double blind, placebo controlled trial conducted by Chadwick, et al., gabapentin demonstrated a significant reduction in seizure frequency compared to placebo in patients with refractory complex partial seizures. Similarly, a retrospective study by Gidal, et al. evaluated gabapentin as an add on therapy and reported a reduction in seizure frequency and improved seizure control in patients with refractory epilepsy [3].
The favorable safety profile of gabapentin further supports its use in epilepsy management. The most commonly reported adverse effects of gabapentin include dizziness, somnolence, and mild gastrointestinal disturbances. Serious adverse events are relatively rare, making gabapentin generally well tolerated by patients with epilepsy [4].
Despite the accumulating evidence supporting the effectiveness of gabapentin, it is important to consider individual patient characteristics, epilepsy syndrome, and potential drug interactions when determining the most appropriate treatment regimen. Additionally, further research and clinical trials are needed to optimize the use of gabapentin in epilepsy management and assess its long term effects [5].
Types of epilepsy
Epilepsy encompasses a diverse range of seizure disorders, classified into different types based on various factors including seizure characteristics, underlying causes, and brain regions involved. Understanding the different types of epilepsy is crucial for accurate diagnosis and appropriate treatment selection (Figure 3). The following provides a summary of some common types of epilepsy [6].
Figure 3: Evaluation of epilepsy in children.
Focal (partial) epilepsy: Focal epilepsy, also known as partial epilepsy, originates from a specific region of the brain. Seizures in focal epilepsy are characterized by abnormal electrical discharges localized in a specific area, which may result in motor, sensory, autonomic, or cognitive symptoms. Focal seizures can be further classified as focal aware seizures (formerly known as simple partial seizures) or focal impaired awareness seizures (formerly known as complex partial seizures) (Figure 4). The distinction between the two is based on the individual's level of awareness during the seizure [7].
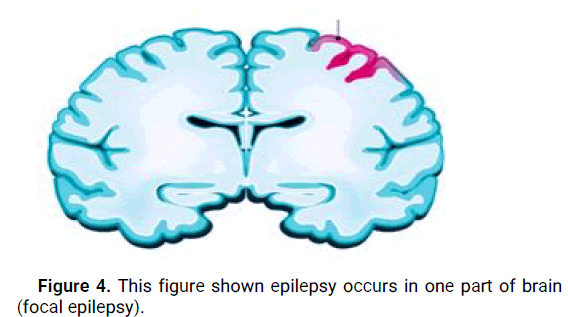
Figure 4: This figure shown epilepsy occurs in one part of brain (focal epilepsy).
Generalized epilepsy: Generalized epilepsy involves seizures that originate from and involve both cerebral hemispheres of the brain from the onset. These seizures typically affect the entire brain, resulting in widespread symptoms such as loss of consciousness, convulsions, and bilateral motor manifestations (Figures 5 and 6). Types of generalized epilepsy include generalized tonic-clonic seizures (formerly known as grand mal seizures), absence seizures (formerly known as petit mal seizures), myoclonic seizures, and atonic seizures [8].
Figure 5: This picture showed epilepsy affect whole brain (generalized epilepsy).
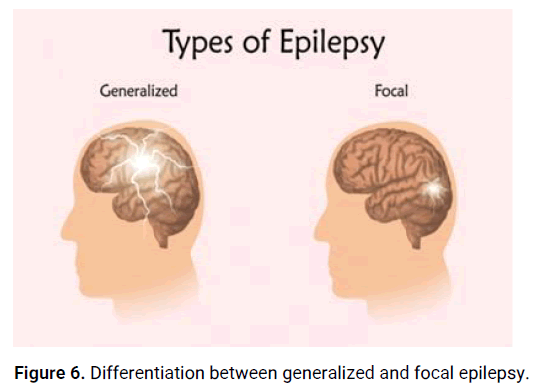
Figure 6: Differentiation between generalized and focal epilepsy.
Symptoms of epilepsy
Here are some common symptoms associated with epilepsy:
Seizures: Seizures are the hallmark symptom of epilepsy. They can manifest in various ways, including convulsions, sudden loss of consciousness, staring spells, repetitive movements, or unusual sensations.
Aura: Some individuals with epilepsy may experience an aura before a seizure occurs. An aura is a warning sign that can manifest as a strange smell, taste, visual disturbance, or an intense feeling of fear.
Loss of awareness: Certain types of seizures, such as absence seizures, can cause a temporary loss of awareness. During these seizures, the person may appear to be staring blankly into space and may not respond to stimuli.
Uncontrolled movements: Seizures can involve involuntary movements of the body, such as jerking of the limbs, twitching, or repetitive motions.
Sensory changes: Epileptic seizures can affect the senses, leading to abnormal sensations such as tingling, numbness.
Emotional or cognitive changes: Some individuals may experience mood changes, confusion, memory lapses, or difficulty speaking during or after a seizure.
Physical symptoms: Seizures can sometimes cause physical symptoms like headaches, dizziness, fatigue, or loss of bowel or bladder control (Figure 7).
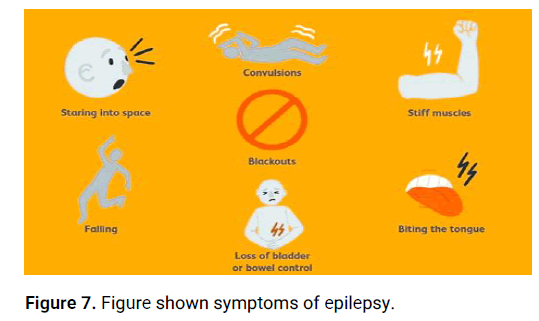
Figure 7: Figure shown symptoms of epilepsy.
Antiepilepsy
Antiepileptic Drugs (AEDs), also known as antiseizure medications or antiepilepsy drugs are a class of medications used to manage and control seizures in individuals with epilepsy. They work by suppressing or reducing the abnormal electrical activity in the brain that leads to seizures. Antiepileptic drugs are a cornerstone of epilepsy treatment and play a crucial role in seizure prevention and improving the quality of life for individuals with epilepsy [9].
These medications act through various mechanisms to achieve their antiepileptic effects. Some common mechanisms of action include:
Enhancement of inhibitory neurotransmission: Many AEDs enhance the activity of the inhibitory neurotransmitter Gamma- Aminobutyric Acid (GABA) in the brain. GABA inhibits excessive neuronal activity, helping to prevent seizures. Examples of AEDs that act through this mechanism include benzodiazepines, barbiturates, and gabapentinoids.
Inhibition of excitatory neurotransmission: Some AEDs inhibit the activity of excitatory neurotransmitters, such as glutamate, which can contribute to seizure generation. By reducing excitatory neurotransmission, these medications help to decrease the likelihood of seizures. Examples of AEDs that act through this mechanism include topiramate and felbamate [10].
Modulation of ion channels: Certain AEDs target ion channels in the brain, such as sodium channels or calcium channels, which are involved in generating and propagating electrical signals in neurons. By modulating these channels, AEDs can help stabilize neuronal activity and prevent abnormal electrical discharges associated with seizures. Examples of AEDs that act through this mechanism include phenytoin and lamotrigine [11].
Modulation of neurotransmitter release: Some AEDs affect the release and balance of various neurotransmitters, such as GABA, glutamate, and others, which play essential roles in neuronal communication. By modulating neurotransmitter release, these medications can help regulate brain activity and reduce the likelihood of seizures. Examples of AEDs that act through this mechanism include valproic acid and levetiracetam [12].
Mechanism of action of gabapentin
The precise mechanism of action of gabapentin is not fully understood (Figure 8). However, it is believed to involve modulation of calcium channels and GABAergic neurotransmission in the central nervous system. Here is a simplified description of the proposed mechanism of action of gabapentin:
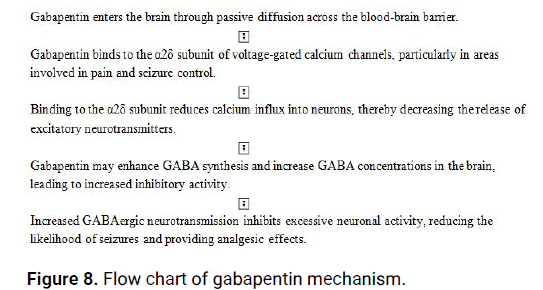
Figure 8: Flow chart of gabapentin mechanism.
Pharmacokinetics of gabapentin
Understanding the pharmacokinetics of gabapentin is important for determining the appropriate dosing regimen and ensuring optimal therapeutic effects. Here are the key aspects of the pharmacokinetics of gabapentin:
Absorption: Gabapentin is rapidly and almost completely absorbed from the gastrointestinal tract after oral administration. However, its absorption is limited by an active transport mechanism, which can result in dose dependent absorption. Food intake does not significantly affect the extent of gabapentin absorption but may delay the time to reach peak plasma concentration.
Distribution: Gabapentin exhibits moderate plasma protein binding, with approximately 30% binding to plasma proteins. The drug has a relatively large volume of distribution, indicating extensive tissue distribution. Gabapentin crosses the blood-brain barrier and reaches therapeutic concentrations in the Central Nervous System (CNS).
Metabolism: Gabapentin is primarily eliminated unchanged by renal excretion. It undergoes minimal hepatic metabolism, and the liver plays a minor role in its elimination. There are no known clinically significant drug interactions involving hepatic enzymes.
Elimination: Gabapentin is eliminated from the body primarily through renal excretion. It is mainly excreted in the urine as unchanged drug, with approximately 90% of the administered dose being recovered in the urine within 24 hours. Renal clearance of gabapentin is directly proportional to creatinine clearance, so dose adjustment is necessary for individuals with impaired renal function.
Half-life: The elimination half-life of gabapentin is generally reported to be approximately 5 to 7 hours in individuals with normal renal function. The half-life may be prolonged in individuals with impaired renal function, requiring dosage adjustment based on renal function.
Dose: The appropriate dose of gabapentin can vary depending on the condition being treated, the patient's age and weight, renal function, and other individual factors. It is essential to follow the prescribed dose provided by a healthcare professional. However, I can provide some general information about the dosing guidelines for gabapentin.
For the treatment of epilepsy in adults and adolescents (12 years of age and older), the usual starting dose of gabapentin is 300-900 mg per day, divided into three doses. The dose can be gradually increased based on individual response and tolerability, typically up to a maximum daily dose of 3600 mg.
For the management of post herpetic neuralgia (a complication of shingles), the initial dose of gabapentin is often 300 mg taken once a day on day 1, 300 mg twice a day on day 2, and 300 mg three times a day on day 3. The dose may be titrated up to a maximum of 3600 mg per day based on individual response and tolerability.
It's important to note that these dosing guidelines are general recommendations and may vary depending on the specific condition being treated and individual patient factors. Dosage adjustments may be necessary for patients with impaired renal function, as gabapentin is primarily eliminated through the kidneys. Renal function should be assessed, and the dose should be modified accordingly.
Always consult a healthcare professional or refer to the prescribing information for specific dosing instructions for gabapentin, as they can provide the most accurate and up-todate information based on the individual's needs and medical condition.
Adverse effect of gabapentin
Gabapentin, like any medication, can potentially cause adverse effects in some individuals. It is important to be aware of these potential adverse effects and consult a healthcare professional if you experience any concerning symptoms. Here are some common adverse effects associated with gabapentin use:
Central nervous system effects
• Dizziness
• Drowsiness
• Fatigue
• Headache
• Coordination difficulties
Gastrointestinal effects
• Nausea
• Vomiting
• Diarrhea
Peripheral edema
Swelling of the extremities, such as the hands, feet, or ankles
Weight gain
Some individuals may experience weight gain while taking gabapentin.
Behavioral changes
• Mood swings
• Depression
• Anxiety
• Irritability
Visual disturbances
• Blurred vision
• Double vision
Allergic reactions
• Skin rash
• Itching
• Hives
• Swelling of the face, lips, tongue, or throat (angioedema).
Diagnosis of epilepsy
Here is a general outline of the diagnostic process for epilepsy, along with some reputable references for further information:
Medical history and physical examination: The doctor will conduct a detailed interview to gather information about the patient's symptoms, medical history, family history, and any potential triggers or events preceding the seizures. A physical examination may also be performed to assess overall health.
Electroencephalogram (EEG): EEG is a common test used to record the electrical activity in the brain. It helps detect abnormal brain wave patterns that may indicate epilepsy. Sometimes, prolonged EEG monitoring or video EEG monitoring is used to capture seizures during the test.
Imaging tests: Imaging techniques such as Magnetic Resonance Imaging (MRI) or Computed Tomography (CT) scans are performed to identify any structural abnormalities or brain lesions that could be causing the seizures.
Blood tests: Blood tests may be conducted to check for underlying conditions or metabolic disorders that can contribute to seizures.
Additional diagnostic tests: In some cases, additional tests such as neuropsychological assessments, genetic testing, or sleep studies may be recommended to further evaluate the individual's condition.
Gabapentin has been established as an effective treatment for epilepsy. Numerous clinical studies and research have shown its efficacy in reducing seizure frequency and improving seizure control in individuals with epilepsy.
Gabapentin is an anticonvulsant medication that works by modulating the activity of certain neurotransmitters in the brain, particularly by enhancing the inhibitory effects of Gamma- Amino Butyric Acid (GABA). This helps to stabilize abnormal electrical activity in the brain, which is a characteristic feature of epilepsy.
Studies have demonstrated the effectiveness of gabapentin as an adjunctive treatment for various types of seizures, including partial onset seizures and generalized tonic-clonic seizures. It has been shown to significantly reduce seizure frequency, improve seizure control, and enhance the quality of life for individuals with epilepsy.
Furthermore, gabapentin is generally well tolerated, with a favorable side effect profile compared to some other antiepileptic drugs. Common side effects may include dizziness, drowsiness, and mild gastrointestinal disturbances.
It's important to note that the use of gabapentin for epilepsy should be determined by a healthcare professional, who will consider various factors such as the individual's specific seizure type, medical history, and other medications being taken.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Gupta Y. "Gabapentin as an Effective Treatment for Epilepsy". J Neuro Neurophysio, 2023,14(5), 1-4.
Received: 21-May-2023, Manuscript No. JNN-23-99416; Editor assigned: 23-May-2023, Pre QC No. JNN-23-99416 (PQ); Reviewed: 06-Jun-2023, QC No. JNN-23-99416; Revised: 21-Jul-2023, Manuscript No. JNN-23-99416 (R); Published: 28-Jul-2023, DOI: 10.35248/2155-9562.23.14(5).371
Copyright: © 2023 Gupta Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.