Review Article - (2020) Volume 11, Issue 6
Community Acquired Bacterial Meningitis (CABM) is a leading cause of morbidity and mortality in Africa, affecting over 2.8 million people annually. The African continent bears the largest burden of CABM with six out of the ten countries having the highest incidence and mortality. This systematic review set out to determine the leading causes of CABM in Africa, the distribution of etiologies across regions, and the extent of antimicrobial resistance of identified microbial agents. Methodology: The databases PubMed and Embase were queried with key search words “bacterial meningitis in Africa”, “community acquired bacterial meningitis” or “meningitis in Africa” published between 1990 and 2019. Using the PRISMA guidelines for systematic reviews, the search retrieved 112,972 articles. We included studies reporting primary patient data with confirmed CABM. Results: Sixty-four studies were analyzed which were conducted in 23 African countries between 1990 and 2019. Cumulatively, we analyzed 118,716 suspected cases of CABM, with confirmed CABM in 34,593 (29%) cases. Streptococcal pneumoniae (Spn) was found to be the most prevalent cause of disease at 12.4% (CI 11.1%-13.6%), Neisseria meningitidis (Nmn) at 8.1% (CI 7.1%-9.2%) and Hemophilus influenza (Hib) at 4.0%(CI 3.6%-4.3)%. The etiologies varied by region, with Nmn being most prevalent in West and South Africa regions and Spn in the rest of the continent. Salmonella prevalence was 0.35%(0.22%-0.47%) representing 16% of the total cases in East Africa but less than 1% in other regions. Of the 64 studies reviewed, 29 (45%) reported on antimicrobial resistance. Resistance was found to be highest in ampicillin 2.7% (CI 2.1%-3.3%) and gentamycin 2.75%(2.09-3.40). Conclusions: We found a gross paucity of information on CABM in Africa despite baring the highest burden of disease. Spn and Nmn are still the leading causes of disease, and etiology varies markedly with region. Antimicrobial resistance of ampicillin and gentamycin are widespread despite being the commonest drugs used in the treatment of CABM in Africa.
Bacterial meningitis • Antimicrobial resistance • Community acquired bacterial meningitis • Africa
Over 2.8 million people suffer from bacterial meningitis (BM) globally with the African continent bearing the largest burden of disease [1]. Over the last decade the roll out of vaccination programs, improved diagnostic testing, and early treatment has led to marked reduction in incidence and mortality of CABM. Globally, the mortality rate of CABM is 14% with treatment. In resource limited settings including areas of the African continent, mortality rates are significantly higher than those of more developed countries. While Africa bears the largest burden of CABM, the incidence varies widely by region, for example, Rwanda reports a very low incidence of 0.75 per 100,000 while others, such as South Sudan, report much higher incidence of 204.7 per 100,000 persons. Analysis of current CABM prevalence, incidence and microbial resistance by African region may direct region-specific disease management.
Most cases of BM globally have been attributed to Haemophilus Influenzae (Hib), Neisseria Meningitidis (Nmn) and Streptococcus Pneumoniae (Spn). Over the last decade the roll out of vaccination programs, improved diagnosis, and early treatment for BM against these three etiologies has led to marked reduction in incidence and mortality of BM, especially in developed countries [1]. Africa has lagged in these efforts despite action by the World Health Organization (WHO) and the Global Alliance for Vaccines and Immunization (GAVI) which have spearheaded efforts to eliminate meningitis in developing countries [2]. Additionally, forms of Streptococcus, Enterococcus and Salmonella species have been identified as potentially new leading causes of BM in some countries [3]. Other factors contributing to the continued high mortality in Africa is antimicrobial agent resistance which has been reported against penicillin’s and aminoglycosides.
There is still significant work to be done to reduce burden of BM in African countries, and it is important to understand patterns in disease etiology and bacterial resistance to target elimination efforts. This systematic review provides an overview of CABM prevalence and etiological distribution. We also highlight the effects of vaccination on the prevalence of the leading etiologies of CABM as well as the burden of antimicrobial resistance in Africa differentiated by region [4-10].
Using the Cochrane collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines of systematic reviews, we systematically searched PubMed and EMBASE databases using key search terms “bacterial meningitis in Africa”, “community acquired bacterial meningitis”, “etiology of bacterial meningitis in Africa”, “meningitis in Africa.” between 1990 and 2019.
Inclusion Criteria
We included studies written in English or French between January 1990 and December 2019 and reported primary data on the etiology of CABM in Africa. This included case control studies, cohort studies and cross-sectional studies, both retrospective and prospective in nature. We also reviewed reference lists of meta analyses and systematic reviews to identify articles. Articles that had data on antimicrobial resistance were included in the study [11-20].
Exclusion Criteria
Studies were excluded if they were expert opinions, letters to the editor, editorials, comments, narrative reviews and case reports. We excluded articles that included mycobacteria tuberculosis and cryptococcal meningitis or articles reporting on a single etiology of BM, e.g. studies reporting only meningococcal meningitis or pneumococcal meningitis. As our focus was on CABM, articles on nosocomial BM were excluded.
All articles meeting inclusion criteria were assessed for data by at least two reviewers who were blinded to each other’s findings. Where there was discrepancy between the findings of the two reviewers, a third person adjudicated the results [21-30].
Data Collection and Verification
The following data was collected: study design, study period, study location and setting, and study participants’ demographics. Participants were classified as children when aged 0 to 18 years of age, and adults as 19 years and older. We collected information on the study years and if vaccination against Hemophilus influenza (Hib), Streptococcal pneumoniae (Spn) and Neisseria meningitidis (Nmn) had been implemented regionally at the time of study enrollment. Organisms causing BM were recorded as well as the diagnostic testing that was used. Seasonality and whether the study was carried out during an outbreak was recorded.
When available, data on antimicrobial resistance was also collected and analyzed. Antimicrobial resistance to common antibiotics used for pediatric CABM, including Penicillin G, Ampicillin, Amoxicillin, Gentamycin, Ceftriaxone and Chloramphenicol was recorded [31-40].
Risk of Bias Assessment
We utilized the checklist from the Critical Appraisal Skills Program (CASP) to assess the articles meeting inclusion criteria on design, content and selection of patients relevant to our study. We assessed for bias using an eight-question checklist composed of “yes” or “no” questions, with results scored on a 10 to 15-point scale based on the relevance of the question. The study was then qualified as either high quality (scored >80%), moderate quality (scored 50- 80%), and low quality (scored <50%). All articles that were included in this study scored moderate to high in quality.
Statistical Analysis
Meta-analysis of etiologies was conducted to provide quantitative summaries and integrate results across studies using random effects models. Specifically, we performed analysis for the frequency of common BM etiologies including Spn, Hib, Nmn, Escherichia coli (E. coli), Listeria monocytogenes, Salmonella typhi and non-typhi, Klebsiella, “other Streptococcal” and all the “other organisms”. The analysis was stratified by country and region of Africa, East, Central, West, South and North, as per the United Nation statistical department (Figure 1). The analysis was also stratified according to age into two groups with group 1 below 18 years and group 2 above 18 years. Studies that did not clearly define the ages of the participants were excluded in age distribution related analysis [41-50].
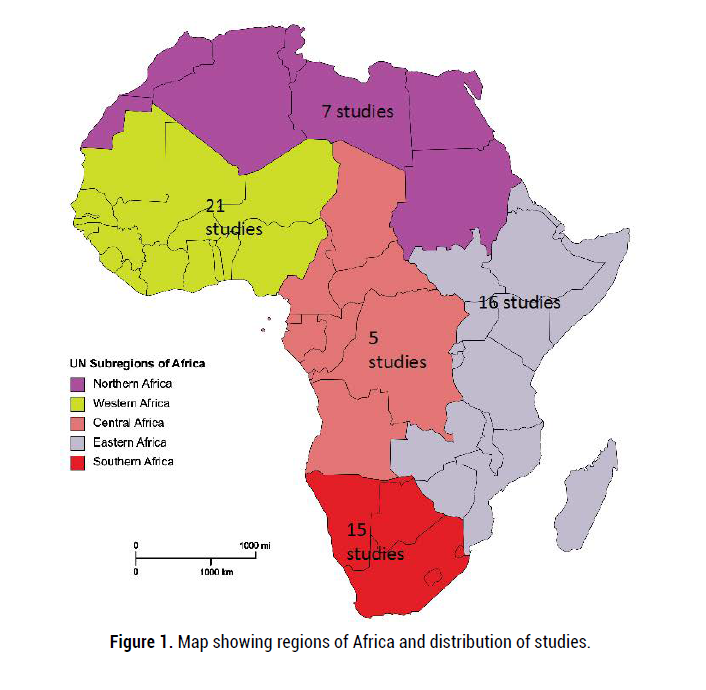
Figure 1. Map showing regions of Africa and distribution of studies.
We compared the percentages of etiologies in subgroups based on study years, whether study was carried out before or after vaccine was introduced and region. We utilized a mixed-effect model, in which the overall effect size for each subgroup is calculated using a random-effect model, and the test for subgroup differences is conducted using a fixed-effect model. In subgroup analysis based on study years, we compared studies that were conducted before 2000 to those studied between 2000 and 2019.We analyzed the changes in infectious etiologies of BM by further grouping studies based on date of publication as pre-2000 and 2000 to 2019. Similar sub-analyses were performed for studies based on whether they occurred in the pre-vaccination (period before documented introduction of immunization for Hib,Nmn and Spn)or post-vaccination (period after documented introduction of vaccines)era.
In studies with antimicrobial resistance data, resistance was calculated as a percentage of documented pathogens found resistant to common antibiotics.
All organisms that showed complete resistance or intermediate resistance were considered resistant in this study [51-55].
The search yielded 112972 articles of which 64 studies were analyzed, representing 43% of African countries [56-65]. Western region of Africa produced the most studies, 23 (35%) (Figure 2). Thirty-six (55%) studies were completed between 2010-2020 with the 29 (45%) being carried out between 1990-1999. Seasonality was reported in 14 (22%) of the studies.
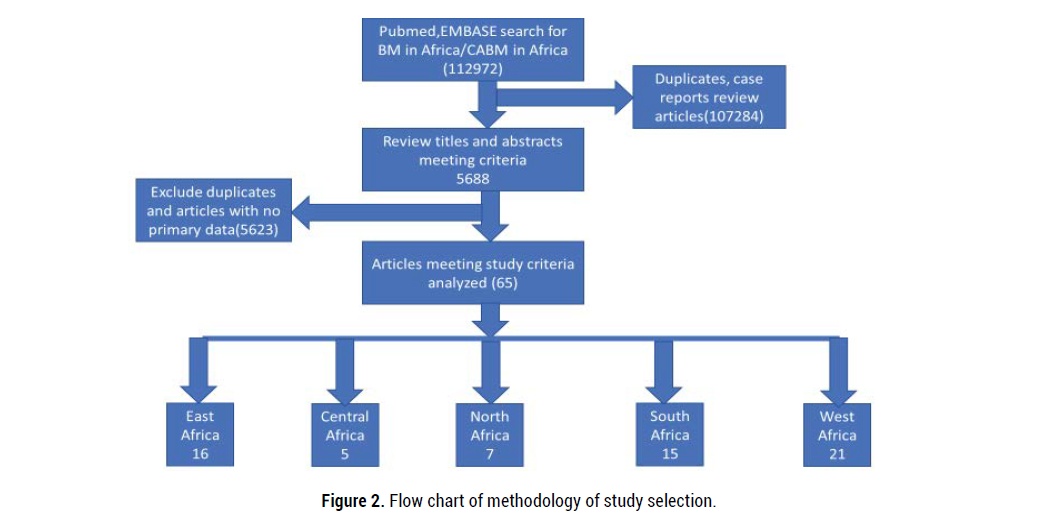
Figure 2. Flow chart of methodology of study selection.
A total of 118,716 people with suspected BM were assessed across the studies, with an organism identified in 34,593 (29%). All studies used CSF culture to confirm diagnosis. Other diagnostic methods that were used together with CSF culture included polymerase chain reaction in 33 (51%) and CSF antigen testing in 11 (17%). Among the confirmed cases, 23,597 (68%) did not report data on age. In 53 studies with data on age, children made up 9506 (83%) of cases (Table 1). Vaccination use was reported in 42 (63%) of the studies.
| Property | N=64 | ||||
|---|---|---|---|---|---|
| Study design | Prospective 42(65%) | Retrospective 13(20%) | Surveillance 9(15%) | ||
| Study region | West 21(32%) | South 15(23%) | East 16(25%) | Central 5(8%) | North 7(11%) |
| Study period | 1990-1999 16(25%) | 2000-2009 23(35%) | 2010-2020 36(55%) | ||
| Vaccination | Pre- vaccination 13(20%) | Post-vaccination 41(63%) | Not indicated 10(17%) | ||
| Diagnostics | CSF culture 64(100%) | PCR 35(51%) | Antigen test 11(17%) | ||
| Seasonality | Dry season 11(17%) | Outbreaks 3(5%) | No seasonality 50(78%) | ||
| Age (N=34,593) | Children(0-18yrs) 9506(28%) | Adults (>18 yrs.) 14904(4%) | Unclassified 23597(68%) | ||
| Antimicrobial resistance | Reported 29(45%) | Not reported 35(55%) |
Table 1. Study demographics
Etiology
In Africa, Spn, Nmn and Hib were the reported leading causes of CABM (Table 2). The prevalence of these organisms varies by region, with Spn being the most prevalent in all regions except for the West. In the West region, Nmn is the leading cause of CABM. South, Central and East Africa reported an increased prevalence of Salmonella (Table 3).
| Organism | Overall % | Pre-vaccination% | Post-vaccination % |
|---|---|---|---|
| Spn | 12.36[11.12- 13.61] | 9.97 (8.02-11.91) | 11.98 (10.16-13.80) |
| Nmn | 8.13[7.06-9.20] | 8.90(7.20-10.60) | 7.95 (6.24-9.67) |
| Hib | 3.97[3.60- 4.33] | 7.59(6.11-9.07) | 2.18 (1.85-2.51) |
| Salmonella | 0.35[0.22- 0.47] | 0.68(0.28-1.09) | 0.21 (0.08-0.33) |
| Listeria | 0.00[0.00-0.02] | 0.00 (0.00-0.02) | 0.00 (0.00,0.02) |
| E. coli | 0.12[0.05- 0.19] | 0.25(0.16-0.34) | 0.06 (0.00-0.14) |
| Klebsiella | 0.12[0.05- 0.19] | 0.14 (0.00-0.32) | 0.06 (0.00-0.12) |
| Other strep | 0.63[0.47- 0.79] | 0.56 (0.23-0.90) | 0.82 (0.59-1.05) |
| Others | 0.26[0.21- 0.32] | 1.29(0.80-1.78) | 0.07 (0.03-0.11) |
Table 2. Prevalence of different etiologies in Africa
| Organis m | Central | East | North | South | West | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence% | CI% | Prevalence% | CI% | Prevalence% | CI% | Prevalence% | CI% | Prevalence% | CI% | |
| SPN | 19.52 | (8.83-30.21) | 4.28 | (3.29-5.28) | 18 | (9.47-26.62) | 18.21 | (13.29-23.14) | 11.44 | (8.81-14.06) |
| NMN | 5.79 | (1.96-9.61) | 0.12 | (0.00-0.26) | 5.39 | (2.04-8.75) | 11.59 | (0.00-27.08) | 11.92 | (9.02-14.82) |
| HIB | 7.36 | (4.03-10.68) | 1.66 | (1.05-2.26) | 10.8 | (4.59-16.91) | 13.49 | (10.74-16.24) | 2.48 | (2.05-2.90) |
| Salmonella | 0.98 | (0.00-2.54) | 0.85 | (0.40-1.30) | 0.15 | (0.00-0.35) | 0.55 | (0.19-0.92) | 0.03 | 0.00-0.06 |
| Listeria | 0 | (0.00-0.43) | 0 | (0.00-0.04) | 0 | (0.00-0.02) | 0.01 | (0.00-0.09) | 0 | (0.00-0.02) |
| E coli | 0 | (0.00-0.43) | 0.2 | (0.00-0.43) | 0.35 | (0.00-0.84) | 0.06 | (0.00-0.16) | 0.1 | (0.00-0.20) |
| Klebsiella | 0 | (0.00-0.43) | 0.08 | (0.00-0.16) | 0.38 | (0.00-0.77) | 0.26 | (0.04-0.49) | 0.04 | (0-0.09) |
| Other strep | 1.09 | (0.21-1.98) | 0.66 | (0.25-1.07) | 0.52 | (0.00-1.08) | 2.18 | (1.26-3.10) | 0.23 | (0.08-0.37) |
| Others | 0 | (0.00-0.07) | 0.69 | (0.34-1.03) | 6.07 | (3.88-8.27) | 1.7 | (1.14-2.25) | 0.11 | (0.06-0.16) |
Table 3. Distribution of etiology by region
Comparing studies performed in 1990 to 1999 with those in 2000 to 2019, all etiologies of CABM declined in prevalence (Table 2). Nmn was the most prevalent etiology16.03% (CI 0.0-33.15%) followed by Hib and Spn during 1990 to 1999 (Table 4). This, however, changed from 2000 to 2020, where Spn was reported as the leading cause of CABM. In the post-vaccination period, Spn increased in prevalence from 9.97% (CI 8.02%-11.91%) to 11.98% (CI 10.16%-13.80%). Hib had the largest positive response to vaccination with a 50% reduction in prevalence. Of those organisms with no current vaccine (e.g. Salmonella), there was a decreased prevalence of CABM in the post-vaccination years in all organisms except for “other Streptococcal” organisms.
| Study period | 1990-1999 Prevalence (C.I) % | 2000-2020 Prevalence (C.I) % | p value |
|---|---|---|---|
| Spn | 12.58 (8.47-16.70) | 10.81 (9.42-12.21) | 0.43 |
| Nmn | 16.03 (0.00-33.15) | 7.62 (6.38-8.85) | 0.34 |
| Hib | 14.61 (10.11-19.12) | 2.50 (2.16-2.83) | <0.0001 |
| Salmonella | 0.66 (0.05-1.28) | 0.31 (0.16-0.47) | 0.28 |
| Listeria | 0.03 (0.00-0.15) | 0.00 (0.00-0.02) | 0.55 |
| E. coli | 0.40 (0.03-0.77) | 0.11 (0.03-0.19) | 0.14 |
| Klebsiella | 0.10 (0.00-0.26) | 0.11 (0.03-0.17) | 0.97 |
| Other strep | 0.47 (0.03-0.91) | 0.76 (0.56-0.97) | 0.24 |
| Others | 2.53 (1.50-3.55) | 0.14 (0.09-0.18) | <0.0001 |
Table 4. Change of etiology in two time periods (1990-1999 and 2000-2020)
Antimicrobial Resistance
Twenty-nine (45%) studies reported on antimicrobial resistance. Resistance was found to be highest against ampicillin 2.7% (CI 2.1%-3.3%) and gentamycin with a prevalence of 2.7% (CI 2.1%-3.4%). Amoxicillin with prevalence of 1.0% (CI 0.5%-1.5%) and ceftriaxone 0.14% (0.01%-0.27%) demonstrated the least resistance (Figure 3). Regional variance was noted with the Southern region reporting antimicrobial resistance against all the reviewed antibiotics, and Central Africa reporting resistance only to penicillin. Generally, resistance to ceftriaxone was minimal except in the East region which reported a resistance rate of 18% (Figure 4).
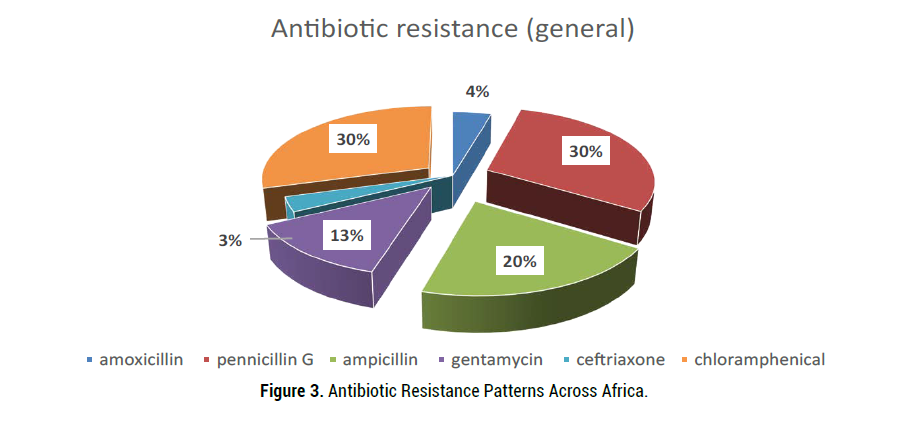
Figure 3. Antibiotic Resistance Patterns Across Africa.
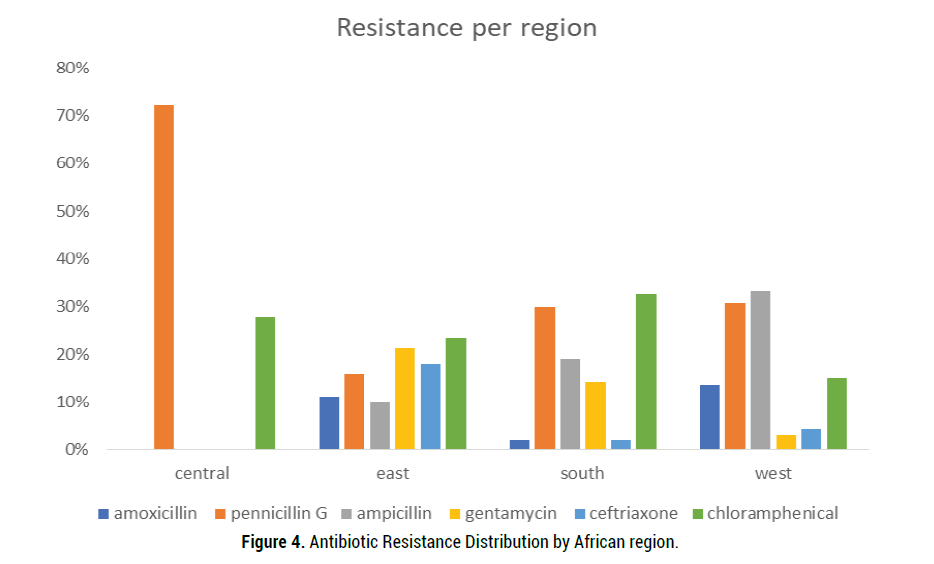
Figure 4. Antibiotic Resistance Distribution by African region.
To the best of our knowledge, this is the first systematic review of the etiological distribution of CABM in Africa. This review found that while Spn, Nmn and Hib were the leading causes of BM in Africa over the last 30 years. We found that children with BM carry the largest burden of disease (86%) compared to adults. This is verified by other studies that have reported more than 50% burden of disease to children.
We found that from 1990 and 1999, Hib was the leading cause of CABM in Africa closely followed by Spn and Nmn. The prevalence of Hib reduced by over 50% between 2000 and 2020 to become the third leading cause of BM in Africa. Previous global reviews reported similar findings; Hib, Spn and Nmn were the leading cause of BM before 2000. However, Hib has remained one of the three leading causes of meningitis disease burden in Africa from 2000 to 2020 [66]. The continued prevalence of Hib in Africa may be due to the vaccine implementation practices including lack of vaccines and frequent stock outs. This is a multifactorial issue and may be due to the lack of funding for vaccines, inadequate implementation of programs and poor infrastructure making transportation to the health facilities difficult.
In this meta-analysis, Spn was found to be the leading cause of CABM in Africa. Similarly, Spn remains the leading cause of BM globally. However, this study differs from other studies that have reported a decrease in Spn prevalence, while we noted an increase in Spn prevalence in the post-vaccination period. Smaller studies have reported an increase in non-vaccine type Spn, which may be driving the increased Spn prevalence that we observed though further studies are required [67].
While there has considerable efforts by the World Health Organization and Global Vaccination Alliance to increase vaccination, Africa still lags in implementation of these preventative measures. We report a reduction in the prevalence of the three leading etiologies of BM, though the change in prevalence we report is much lower compared to other studies. The GBD study reported global decline of the three leading organisms and an increase in “other” organisms, and the latter became the leading cause of BM in 2017. Conversely, our study reports “other” causes which included unspecified gram-positive cocci, pseudomonas, and any other organisms not analyzed individually as the least prevalent. This disparity is possibly due to the grouping of the data. In our study we collected individual data on 8 organisms while the GBD study collected on the 3 leading causes and grouped all other organisms into one. The disparity could also be due to the reduced vaccination efforts and testing capabilities in Africa compared to more developed countries.
Interestingly, there was variation in the leading causes of CABM among different regions of Africa. Spn was the leading cause of BM in East, Central, South and North African regions, whereas Nmn was found to be leading cause of disease in the West region. We found that while the three leading causes of disease were Spn, Nmn and Hib in most of the regions, the East region had Spn, Hib and Salmonella as the leading causes of BM. East Africa also had a higher prevalence of E. coli compared to other regions. A recent systematic review found higher prevalence of E. coli in Africa compared to other continents but did not delineate this by regions. This may be attributed to different cultural and hygienic practices.
In regard to antibiotic resistance, very few studies reported on antimicrobial resistance 45% of our entire dataset. Despite this our study still found that 30% of identified etiologies showed resistance to commonly used antimicrobial agents. Ampicillin and gentamycin, which are the recommended first line treatment for BM, particularly in neonates [68], had the highest reported resistance patterns followed closely by penicillin [26, 69, 70]. The unregulated use of antibiotics in Africa is likely a key contributor to the widespread antimicrobial resistance. This review highlights the need for more data on antimicrobial resistance in Africa, and the need for better systems to monitor and regulate antibiotic use to appropriately address the global burden of antimicrobial resistance [71]. These recommendations should be regionally specific, as the common etiologies of BM and resistance patterns varied widely between regions. More studies are required to identify these patterns of distribution to improve outcomes and guide the development of effective treatment protocols.
The strength of this study is its focus on studies in Africa which is the biggest burden of CABM. The regional analysis of the etiology clearly defines the distribution of etiologies and such gives clear analysis of which organisms should be focused on for particular images. The 30- year analysis provides a snapshot of changes in CABM in Africa and as such provides an analysis of strides made over the years. The limitations of this study include exclusion of studies which included TB may have excluded important data sets, we used only two search engines and this could have limited our access to the data required. We also did not account for the time of introduction of vaccine to time of study and as such analysis of post vaccination may have been skewed if study was carried out in exact time of vaccination.
While several advances have been made towards the prevention, diagnosis and management of BM, the impact of these advances has not been well documented. Over 45% of the studies did not use PCR or antigen testing, which have been reported to have over 97% sensitivity compared to the less than 50% sensitivity reported for CSF culture and gram stain [72, 73]. Given the lack of sensitivity in testing modalities, the prevalence and burden of BM in Africa may not be accurately documented. Understanding the true scope and burden of BM in Africa is contingent on the improvement of diagnostic facilities. This may also identify the other causes of BM that are contributing to an increased prevalence of disease.
There remains a paucity of information on the true burden of CABM in Africa. It is imperative that we work to improve the diagnostic capabilities in Africa. There is a great need to regionally address antimicrobial resistance and treatment guidelines, in hopes of better outcomes and less health disparities.
Kiran Thakur is supported by the National Institute of Health, NINDS K23 NS105935-01 and NIH/NICHD 1R01HD074944-01A1
None declared.
Received: 17-Jul-2020 Published: 29-Sep-2020
Copyright: © 2020 Vivian S Namale, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.