Case Report - (2025) Volume 11, Issue 1
Introduction: Molecular testing has become standard of care in the treatment of Non-Small Cell Lung Cancer (NSCLC). Active driver mutations will often guide therapies, especially in the advanced setting, in which resistance is a likely event. With increasing molecular testing results, it is unclear how to effectively prioritize them to structure each next line of treatment. Herein, we report on a case with a MET exon 14 skipping mutation who developed resistance after treatment with a type 1b targeted TKI.
Case report: Our patient presented as a 76-year-old Hispanic female with newly diagnosed stage IV NSCLC adenocarcinoma. She was found to have a MET exon 14 skipping mutation and was initiated on capmatenib in accordance with the geometry trial. She had a partial response with resolution of her symptoms for about 6 months post therapy initiation and subsequently progressed. Molecular testing showed a MET D1228N resistance and a CCDC6 RET fusion mutation, along with numerous Variants of Unknown Significance (VUS). She was switched to selpercatinib and progressed after a month on treatment. An analysis of variants combined with a high CPS score indicated immunotherapy treatment that the patient has since started.
Conclusion: After treatment with targeted therapies, resistance mechanisms are found with increasing frequency. Herein, we present a patient who developed a classic MET D1228N resistance mechanism and RET fusion, bypassing resistance mechanism. An analysis of molecular variants provided novel insight into the VUS, RBM10, to support immunotherapy. This case demonstrates the need to look for actionable information beyond key findings to determine next therapeutic steps.
MET exon • Hispanic female • Immunotherapy • Selpercatinib • Capmatenib
Molecular diagnostics has become Standard of Care (SOC) directing the management of Non-Small Cell Lung Cancer (NSCLC).
Targeting driver mutations with Tyrosine Kinase Inhibitors (TKI’s) has been utilized since 2003 when Gefitinib demonstrated efficacy on heavily pretreated patients [1]. Since then, an array of targeted TKI’s have proven effective in treating NSCLC. However, molecular resistance mechanisms often occur after TKI therapy. These resistance mechanisms hold opportunities for subsequent lines of treatment, but interpretation is challenging with the compendium of testing results. It is therefore imperative to report on the real-world efficacy of targeted agents and gather data regarding resistance mechanisms to hypothesize in real-time ways to manage patients for an oftentimes near unending next line of treatments. To underscore this imperative, we report a patient with a Met exon 14 skipping mutation and molecular diagnostics revealing a VUS gene mutation as key, supporting immunotherapy as a next option.
Our patient presented with de novo metastatic NSCLC with adenocarcinoma at age 76. She was diagnosed at a hospital in Mexico incidentally while being treated for a severe COVID-19 pneumonia in 02/2023. She underwent a segmentectomy with pleural biopsy in 05/2022 and moved to the US for further treatment.
Pathology from her segmentectomy and pleural biopsy demonstrates a 3 cm moderately differentiated adenocarcinoma, grade 2 with lymphovascular invasion, and pleural metastasis. Her PET CT (Figure 1) demonstrated advanced left sided disease, pleural thickening and pleural effusion.
Figure 1. PET CT imaging over time. (A): Initial staging PETCT scan on May 24 2022; (B): Interim PETCT scan on July 19 2022; (C): PETCT scan after rapid progression after initiation of Selpercatinib April 26 2023.
Owing to insurance and tissue originating outside the country, molecular testing was unavailable. A liquid biopsy (Guardant 360) was reported on 05/22/2022 demonstrating mutations in: MET (exon 14 skipping), ARID1A and included Variants of Uncertain Clinical Significance (VUCS), PDGFRA, BRAF and MET P239fs. Due to the MET exon 14 skipping mutation, she was started on Capmatenib 400 mg BID 06/09/2022 [2]. She tolerated treatment well with reported symptom improvement. An interim PETCT scan demonstrated significant partial anatomic and metabolic response. The following three months were unremarkable with low symptom burden and excellent performance status, as she continued on capmatenib.
In 01/2023, she showed clinical signs of progression with weight loss and shortness of breath. A CT scan of her chest demonstrated progression with increasing pulmonary nodules and new right sided disease (Figure 2). A CT guided biopsy was performed on 03/07/2023 to rule out histologic transformation and obtain tissue for molecular testing.
Figure 2. Timeline of case events (progression, molecular testing and treatment). Molecular testing results are shown below the calendar. Main results are shown in black (red highlighted are resistance mutations); results in grey are Variants of Undetermined Clinical Significance (VUCS) from Guardant and Variants of Unknown Significance (VUS) from Foundation One.
A second liquid biopsy reported on 02/05/2023 demonstrating persistent MET exon 14 skipping and acquired new mutations: MET D1228N and CCDC6-RET Fusion. Molecular results from tissue were obtained (03/20/2023) which showed evidence of MET D1228N, and several other mutations, a stable microsatellite status, and low Tumor Mutational Burden (TMB). My Cancer Molecular Information Exchange (myCMIE) was utilized to help dissect the heterogeneous molecular profiling results, including several VUSes, into those with potential therapeutic implication [3]. The patient was referred for clinical trial but was unable to enroll due to funding. She subsequently initiated selpercatinib 120 mg BID on 03/28/2023 for targeted treatment of the RET fusion mutation.
A month after starting selpercaptinib (04/26/2023), she was found to have rapid progression on a PET CT scan demonstrating worsening LAD and enlarging lung lesions. She was then initiated on immunotherapy with pembrolizumab, which was supported by myCMIE analyses as a potential treatment option.
Exon 14 of the MET gene encodes the juxtamembrane domain, a key component for MET degradation. Without exon 14, the MET signaling pathways become overactive, triggering cell proliferation. MET exon 14 mutations occur in 3-4% of patients with NSCLC [4]. Multiple treatments have been developed to target this mutation, including capmatenib, a selective Type 1b TKI. Predictably, after treatment, resistance occur. These mechanisms are diverse and can include, on target mutations impacting the kinase domain and binding, downstream mutations, or parallel receptor signaling alterations. Our patient developed a Met D1228 mutation, an on-target resistance mechanism well reported in the literature. Additionally, she acquired a RET fusion mutation which has not been previously reported in the literature as a resistance mechanism to capmatenib [5].
With the several mutations identified in tissue we utilized the myCMIE application to help focus results with potential therapeutic implication (Figure 3). The analysis starts by searching public databases for genomic profiles similar to the case (Figure 3B) to define Digital Twins (DT) (Figure 3A). The analysis identified 32 case genomic profile matched digital twins using TCGA lung cancer patients (Figure 3C). A subset of 32 (among the 189) un-matched patients balanced on TMB status and gender with the DT positive group were defined as DT negative and used for comparisons. Compared to the DT negative patients, the case profile matched patients had significantly elevated median abundance of Tregs, and a similar median age at diagnosis that was also similar to the case (Figure 3E). Additionally, the case profile matched group had shorter overall and significantly shorter disease-free survival as compared to the un-matched group (Figure 3F). Among the 13 gene mutations reported, only RBM10, a listed VUS, had significantly (p<0.05) lower expression in mutated versus wildtype TCGA lung cancer patients (balanced for TMB (balanced for TMB status, and gender), suggesting a potential Loss of Function (LOF) mutation (Figure 3G). Collectively, the potential LOF of RBM10 and its reported role in tumor development and prognosis due to its negative correlation with Tregs, supports possible implications for monitoring of these markers with treatment. Importantly, silencing RBM10 has been reported to enhance PD-L1 protein levels and a synthetic lethal screen showed inhibition of WEE1 sensitizes RBM10-deficient lung cancer cells, leading to potential expanded treatment with a WEE1 inhibitor and immunotherapy. Indeed, CPS testing results had a 95% score. Altogether, these analyses provided support for immunotherapy as the next line of treatment.
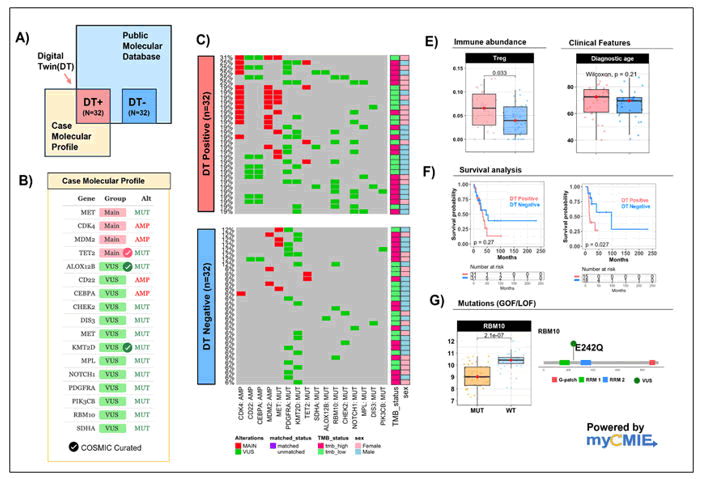
Figure 3. A molecular information exchange analysis on the case genomic profile.
Note: (A): Overview patient-profile connecting analysis to search public database for similar genomic profiles of the input case, powered by the myCMIE application tool; (B): Case molecular profile: Gene symbols, gene groups (main findings in pink, Variant of Unknown Significance (VUS)) in green, and gene alteration type. Genomic mutations curated by COSMIC database (v98) are indicated with a checkmark; (C): Case molecular profile matched to TCGALUAD patients to define n=32 Digital-Twin (DT) positive patients. The case molecular profile un-matched samples are balanced with the case profile-matched samples using TMB status and gender to define n=32 DT negative patients; (E): Boxplots of significant RNASeq derived immune abundance marker and diagnostic age (in years) between DT positive (n=32; in pink) and DT negative (n=32; in blue) patients; (F): Boxplot of overall (left) and disease-free survival (right) between TCGA lung patients case profile-matched and (TMB and gender-balanced) un-matched (DT positive, n=32 in pink; DT negative n=32 in blue); (G): Boxplots of log2(RNA-Seq derived counts +1) expression between RBM10 mutated lung patients (n=34) and (TMB and gender-balanced) RBM10 wildtype patients (n=32).
Patients with actionable driver mutations in NSCLC have many new options for treatment but predictably after mechanisms of resistance occur, treatment decisions become difficult. Understanding the molecular pathways is imperative with choosing the next line of therapy and myCMIE is a powerful tool to assist with this goal.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Auster ME, et al. "MET Exon 14 Resistance in Non-Small Cell Lung Cancer Case Report". Oncol Cancer Case Rep, 2025, 11(1), 1-3.
Received: 25-Aug-2023, Manuscript No. OCCRS-23-111281; Editor assigned: 29-Aug-2023, Pre QC No. OCCRS-23-111281 (PQ); Reviewed: 12-Sep-2023, QC No. OCCRS-23-111281; Revised: 03-Jan-2025, Manuscript No. OCCRS-23-111281 (R); Published: 10-Jan-2025, DOI: 10.35248/2471-8556.25.11(1).004
Copyright: © 2025 Auster ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authora nd source are credited.