Research Article - (2021) Volume 12, Issue 11
Introduction: Facial nerve palsy is caused by a variety of disorders such as herpes zoster, GBS, otitis media, Lyme disease, HIV, etc. In peripheral lesions, the facial weakness that involves the forehead is usually due to a lesion of the ipsilateral facial nerve, but also can be caused by a central lesion (facial nerve nucleus and tract in the pons). Facial diplegia is an extremely rare condition that occurs in about 0.3% to 2.0% of facial palsy cases with the various central or peripheral disorder. The blink reflex is useful in detecting abnormalities in peripheral and central pathways. Methodology: This is a retrospective study, performed at the Neurophysiology unit, HMC, Doha, Qatar. There were 11 patients with bilateral facial weakness who visited for electrodiagnostic studies. Result: Neurologic manifestations including facial diplegia were 72.7% (8), hypo/areflexia were 72.7% (8), facial numbness was 63.6% (7) and lumbar puncture showed CSF albumin-cytological dissociation was 45.5% (5). The most common etiology for facial diplegia was Guillain- Barre syndrome was 81.9% (9). Direct facial nerve stimulation showed 81.8% (9) were bilateral facial nerve low CMAP amplitudes, blink reflex showed 88.8% (8) were bilateral absent responses and EMG showed 55.5% (5) were active denervation in bilateral facial muscles. Conclusion: Facial diplegia is an extremely rare condition which occurs with various central or peripheral diverse etiologies. Electrodiagnostic studies are useful in detecting abnormalities in peripheral and central pathways and prognostic marker in facial diplegia.
Acute motor axonal neuropathy (AMAN) • Facial diplegia (FD) • Nerve conduction study (NCS) • Guillain-Barre Syndrome (GBS) • Amplitude degeneration index (ADI)
Sir Charles Bell first described the facial nerve in the early 1800s [1]. Bell's initial description of facial palsy related to facial paralysis caused by trauma to the peripheral branches of the facial nerve. Bell's palsy, defined as an acute peripheral facial nerve palsy of unknown cause, represents about half of all cases of facial nerve palsy [2,3]. The annual incidence rate is between 13 and 34 cases per 100,000 populations [4]. There is no race, geographic, or gender predilection, but the risk is three times greater during pregnancy, especially in the third trimester or in the first postpartum week [5]. Diabetes is present in about 5 to 10% of patients [6]. Facial nerve palsy may be caused by a variety of disorders including herpes zoster infection, Guillain-Barré syndrome, otitis media, Lyme disease, HIV infection, and others. A peripheral facial palsy is a clinical syndrome of many causes, and herpes simplex virus activation is the likely cause of Bell's palsy is most cases [7]. Herpes zoster is probably the second most common viral infection associated with facial palsy [8]. Recurrent attacks of idiopathic facial palsy on either the ipsilateral or contralateral side have been observed in 7 to 15% of patients [9,10].
A peripheral (lower motor neuron) pattern of facial weakness that involves the forehead is usually due to a lesion of the ipsilateral facial nerve but also can be caused by a central (brainstem) lesion that involves the ipsilateral facial nerve nucleus or facial nerve tract in the pons. Central activation of the facial nerve is both volitional and automatic or emotional in origin, and the facial nerve is the final common pathway. Thus, dissociation of movement of the face to the command from spontaneous movement, as in smiling, indicates an upper motor neuron lesion. Lack of dissociation (absence of both voluntary and spontaneous movement) indicates a lower motor neuron (peripheral) lesion.
Facial diplegia (bilateral facial paralysis) is an extremely rare condition that occurs with various central or peripheral illnesses such as sarcoidosis, Lyme disease, Guillain-Barre Syndrome (GBS), Melkersson Rosenthal syndrome, tuberculous meningitis, leptomeningeal lymphomatosis/carcinomatosis, some neuromuscular junction disorders, and in various muscular dystrophies, etc [11]. It occurs in about 0.3% to 2.0% of facial palsy cases [12]. The electrophysiologic evaluation of facial nerve can be used such as direct facial nerve stimulation, blink reflex, and needle EMG examination. Direct facial nerve stimulation evaluates only the distal segment of the nerve, blink reflex may assess proximal segment of the facial nerve as well as entire blink reflex arc between the trigeminal, brainstem, and facial nerves [13-15]. In general, facial nerve CMAP amplitude of 50 to 75% lower than the contralateral side is associated with a poorer prognosis, long recovery time, and aberrant reinnervation [16]. A small concentric EMG needle should always be used to study facial and trigeminal nerves innervated muscles to look at cranial nerve’s dysfunction. Neuroimaging is warranted if the physical signs are atypical, there is a slow progression beyond three weeks, or if there is no improvement at four months. History of a facial twitch or spasm that precedes facial weakness suggests nerve irritation from the tumor and should also prompt imaging [17].
This is an observational cross-sectional retrospective study, performed at the Clinical Neurophysiology unit, Department of Neurology, HMC, Doha, Qatar, after review and approval by the institutional review board, medical research center (MRC-01-19-239). Eleven patients with clinical bilateral facial paralysis were records, who visited Neurophysiology unit for electrodiagnostic study, during the period from J January 1, 2017 to May14, 2019. Patients with a history of recurrent peripheral facial nerve palsy, previous history of Ramsay hunt syndrome, traumatic facial nerve palsy, upper motor neuron type facial nerve palsy, and presence of cardiac pacemaker (ICD) were excluded. Patients with bilateral facial nerve palsy or facial diplegia either symmetrical or asymmetrical were included. Clinically facial nerve palsy can be classified by the House-Brackman scale from grade 1 to 6. These stages correspond with pathologic findings of neurapraxia (grade 1), axonotmesis (grade 2-3), neurotmesis (grade 4), and partial and complete transection of the facial nerve (grade 5-6). The neurophysiological studies were performed within 2 weeks of the onset of facial diplegia using MEDTRONIC key point V5.03 four-channel amplifier. Direct facial NCS tests were performed with percutaneous stimulation of facial nerve and recording from facial muscles (nasalis or orbicularis oculi) bilaterally. The supramaximal compound muscle action potential (CMAP) was obtained from facial muscles to measure amplitude degeneration index (ADI) using equation; 100-(amplitude affected side/ unaffected side x100). Blink reflex studies were performed with stimulation supraorbital nerve of trigeminal nerve, recorded from orbicularis oculi muscles bilaterally with patient lying down in a quiet room. The evoke muscles action potential (Ipsilateral R1 and R2, and contralateral R2 response) responses were defined as normal, delayed (ipsilateral R1 latency >13 ms and R2 latency >41 ms and contralateral R2 latency >44 ms), and absent (normal blink reflex study as shown in figure 1). Needle EMG examining muscles of frontalis, orbicularis oculi, orbicularis oris, mentalis, and masseter on the affected side. The EMG findings including a degree of insertional activity, spontaneous activity, voluntary activity and recruitment and interference pattern noted. The primary aim of this study is to evaluate electro-diagnostic studies as a prognostic marker for facial diplegia and determine the etiologies of facial diplegia.
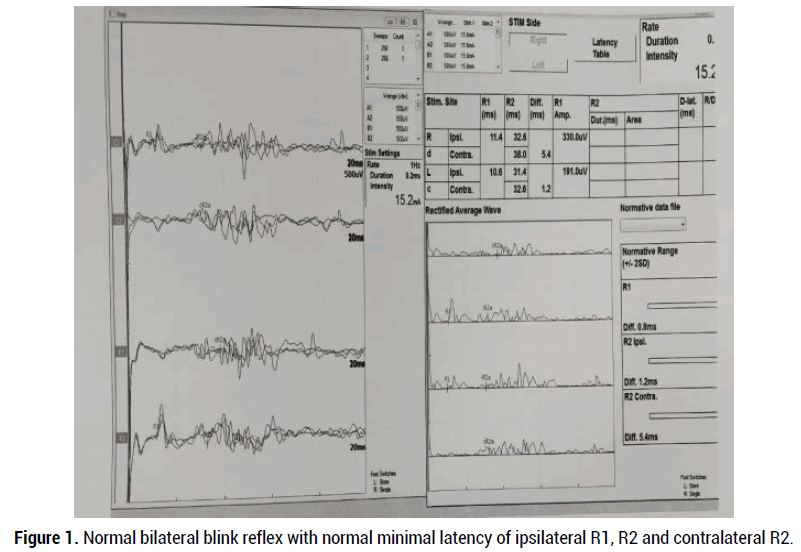
Figure 1. Normal bilateral blink reflex with normal minimal latency of ipsilateral R1, R2 and contralateral R2.
Total of 11 patients with facial diplegia were enrolled, who visited to clinical neurophysiology unit in Hamad General Hospital for electrodiagnostic study including blink reflex. Their demographic as shown in Table 1 and clinical characteristics are as shown in table (2). Their mean age was 36 year (range from 21 to 68 year) and 63% (7) were men and 37% (4) were female. Neurologic symptoms and signs as shown in Table 2 including 72.7% (8) were facial diplegia, 72.7% (8) were hypo/areflexia, 63.6% (7) were facial numbness, 54.5% (6), 36.4% (4) were dysarthria, ataxia, dysphagia and limb numbness each, 27.3% (3) were ophthalmoplegia, 18.2% (2) were UL weakness and 9.1% (1) were LL weakness. In Table 3, lumbar puncture analysis showed 45.5% (5) were high CSF protein level, 45.5% (5) was albumin-cytological dissociation, 18.2% (2) were pleocytosis with <50 neutrophil counts and 9.1% (1) was antiGQ1b positive. In Table 4 shown diagnosis, 81.9% (9) were Guillain- Barre syndrome, 9.1% (1) was lymphomatosis carcinomatosis, 9.1% (1) was trigeminal motor neuropathy and six cases were Guillain- Barre syndrome variant of facial diplegia, one of each case was pharyngeal cervical brachial, AMAN and Miller Fisher syndrome. In Table 5 of electrodiagnostic study, 30% (3) upper limbs nerve conduction study was abnormal, 20% (2) lower limbs nerve conduction study was abnormal, 81.8% (9) direct facial nerve conduction study was abnormal, 88.8% (8) blink reflex study were bilateral absent evokes responses. 55.5% (5) needle EMG examinations were abnormal with active denervation (fib:/PSW) in bilateral facial muscles while one patient showed chronic neurogenic unit in left side muscles of mastication. Repetitive nerve stimulation (RNS) of four patients did not show significant decrement response (>10%). In Table 6, MRI head with gad: showed three patients had bilateral enhancement of the facial nerves and one patient due to lymphoma infiltrates and one patient had atrophy of left muscles of mastication and subtle prominence of the mandibular division of the left trigeminal nerve post-dental extraction. In the Table 7, 72.7% (8) were received IVIG therapy.
| Age(years) | ||
| Mean=36y | (21 to 68 range) | |
| Gender | ||
| Male | 7 | |
| Female | 4 | |
| Nationality | ||
| Indian | 3 | |
| Bangladesh | 1 | |
| Filipino | 2 | |
| Kuwaiti | 1 | |
| Pakistani | 1 | |
| Sudani | 1 | |
| Nigerian | 1 | |
| Syrian | 1 | |
Table 1: Demographics
| Symptoms/signs | ||
| Frequency | ||
| 8 | ||
| Facial diplegia | 72.7% | |
| Hypo/Areflexia | 8 | 72.7% |
| Facial numbness | 7 | 63.6% |
| Dysarthria | 6 | 54.5% |
| Limb numbness | 4 | 36.4% |
| Dysphagia | 4 | 36.4% |
| Ataxia | 4 | 36.4% |
| Ophthalmoplegia | 3 | 27.3% |
| UL weakness | 2 | 18.2% |
| LL weakness | 1 9.1% |
Table 2: Clinical manifestation
| Pleocytosis (22 and 28 neutrophil count) | 2 | 18.2% |
| High Protein (range 0.59 to 1.46) | 5 | 45.5% |
| Albumin cytological dissociation | 5 | 45.5% |
| Low CSF sugar | 0 | 0 |
| Oligoclonal bands+ | 0 | 0 |
| AntiGQ1b+ | 1 | 9.1% |
Table 3: Lumbar Puncture (10 patients performed)
| Underlying Diagnosis | ||
| Frequency | ||
| Guillain- Barre syndrome | 81.9% | |
| Lymphomatosis carcinomatosis | 9.1 9.1 |
|
| Trigeminal motor neuropathy | 9.1 | |
| GBS Variant: - Facial diplegia |
6 | |
| pharyngeal cervical brachial | 1 | |
| Miller Fisher syndrome | 1 | |
| AMAN | 1 | |
Table 4:Diagnosis
| Case# | UL-NCS | LL-NCS | Facial NCS, Amp (mv), DL (ms), ADI | EMG | Blink reflex | RNS | Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | normal | normal | Right=2mv,3.6ms Left=1.3mv,3.6ms ADI=35% |
Insert:/ Spt: activity: +2fib/PSW. Vol: MUAP: reduce IP, recruitment, rapid firing in bil facial muscle. |
Absent bilateral | Normal | Facial diplegia-GBS |
| 2 | normal | normal | Right=1.1mv,3.2ms Left=1.6mv,3.2ms ADI=25% |
Insert/ Spt. activity: +2fib/PSW. Vol: MUAP: no activation facial muscle. |
Absent bilateral | Not performed | Facial diplegia- Lymphomatosis carcinomatosis |
| 3 | normal | normal | Right=0.5mv,3.8ms Left=0.7mv,3.9ms | Insert/ Spt. activity: +2fib/PSW. Vol: MUAP: no activation facial muscle. |
Absent bilateral | Not performed | Facial diplegia-GBS |
| 4 | Abnormal (demyelination) |
Abnormal (demyelination) |
Right=0.5mv,3.4ms Left=0.7mv,3.5ms | Insert/ Spt. activity: normal Vol: MUAP: reduce IP, recruitment, rapid firing in limbs. |
Absent bilateral | Not performed | Facial diplegia-GBS-(AIDP) |
| 5 | Abnormal (axonal) |
Abnormal (axonal) | Right=0.8mv,3.7ms Left=0.3mv,3.5ms | Insert/ Spt. activity: +1fib/PSW. Vol: MUAP: reduce IP, recruitment, rapid firing bil facial muscles |
Not performed | normal | Facial diplegia-GBS-(AMAN) |
| 6 | normal | normal | Right=4.5mv,2.5ms Left=4.5mv,2.2ms (Hx of 2-day facial weakness) |
Insert/ Spt. activity: normal Vol: MUAP: reduce IP, recruitment, rapid firing bil facial muscles |
Absent bilateral | Not performed | Facial diplegia-GBS |
| 7 | Abnormal + spinal accessory nerve (axonal) | normal | Right=0.7mv,3.1ms Left=0.8mv,3.6ms | Insert/ Spt. activity: +1 fib/PSW. Vol: MUAP: no activation |
Not performed | Normal | pharyngeal cervical brachial- GBS |
| 8 | normal | normal | Right=2.4mv,3.7ms Left=2mv,3.4ms | Insert/ Spt. activity: normal Vol: MUAP: reduce IP, recruitment, rapid firing. |
Absent bilateral | Normal | Facial diplegia-GBS |
| 9 | normal | normal | Right= absent Left=0.3mv,3.8ms | Insert/ Spt. activity: +2fib/PSW. Vol: MUAP: reduce IP, recruitment, rapid firing. |
Absent bilateral | Not performed | Facial diplegia-GBS |
| 10 | normal | normal | Right=0.3mv,3.2ms Left=0.7mv,3.9ms. | Insert/ Spt. activity: +2fib/PSW. Vol: MUAP: reduce IP, recruitment, rapid firing in bilateral facial muscle. |
Absent bilateral | normal | MFS-GBS (AntiGQ1b+ve) |
| 11 | Not performed | Not performed | Right=2.5mv,2.8ms Left=2.6mv,2.3ms. | Insert/ Spt. activity: normal Vol: MUAP: reduce IP, recruitment, rapid firing in left masseter chronic neurogenic MUAP |
normal | Not performed | Left axonal motor trigeminal neuropathy |
Table 5: Electrodiagnostic Study
| Case-2: - Bilateral enhancement of the facial nerves in IAC and right intracanalicular segment of facial nerve, possibility of lymphoma infiltrations. |
| Case-6: - Bilateral focal enhancement along the proximal inner canalicular parts of the facial nerves. Case-9: - Bilateral facial nerve enhancement and around conus medullaris and cauda equina. |
| Case-11: - Atrophy and fatty replacement of the left side muscles of mastication, Subtle prominence of the left foramen Ovale with subtle prominence of the mandibular division of the left trigeminal nerve. |
Table 6: MRI head
| Frequency | Percentage (%) | |
| IVIG | 8 | 72.7% |
| Chemotherapy | 1 | 2.4% |
Table 7: Treatment
There were eleven facial diplegia patients enrolled with or without other neurological deficits. The most common neurological sign and symptoms were bilateral facial weakness, hypo or areflexia, facial numbness, bulbar symptoms (dysarthria, dysphagia), limbs numbness, and ataxia. Out of eleven facial diplegia patients, nine patients were Guillain- Barre syndrome (81.9%) including various variants of GBS like facial diplegia, miller fisher syndrome, pharyngeal cervical brachial, and AMAN. All of the patients had an MRI head with contrast, three patients showed bilateral facial nerve enhancement. The most common electrodiagnostic study findings showed low CMAP amplitudes on direct facial nerve stimulation suggested only distal segment of nerve dysfunction, bilateral absent blink reflex suggested of the proximal segment of the motor facial nerve (or sensory trigeminal nerve), and demyelinating pathophysiology and active denervation on needle EMG of facial muscles with normal muscles of mastication also supporting facial nerve peripheral lesion (lower motor neuron). In facial diplegia patients, bilateral greater than 50% to 75% low facial nerve CMAP amplitudes on side-to-side comparison or greater than 50% to 75% amplitude degeneration index (ADI) and active denervation in needle EMG are suggestive of severe axonal loss and poor prognostic marker. This study helps Neurophysiologist and Neurologist in evaluating patients with facial diplegia and localizing lesion either of peripheral (facial and trigeminal nerves) or central (pons and medulla) pathway. In patients with facial diplegia, direct facial nerve stimulation, blink reflex and needle EMG of facial muscles with nerve conduction studies of limbs are diagnostic investigation for pathophysiology and localizing lesion.
Facial diplegia is an extremely rare condition which occurs with various central or peripheral diverse etiologies. In this study, the most common cause of facial diplegia was Guillain- Barre syndrome. Blink reflex, direct facial nerve stimulation and needle EMG of facial muscles are useful in detecting abnormalities in peripheral (trigeminal and facial nerves) or central (pons and medulla) pathways and prognostic marker in facial diplegia.
“None of the authors has any conflict of interest to disclose.”
Liaquat Ali; data collection, data analysis, manuscript writing & review
Adnan Khan; data analysis, manuscript writing, manuscript review
Gholum Adeli; data analysis, manuscript writing, manuscript review
Mohammad Alhatou; manuscript writing, manuscript review
Osama Elalamy ; data analysis, manuscript writing, manuscript review
Fazal Karim; data collection, data analysis
Iqrar A; data collection, manuscript writing, review
The publication of this clinical research fund by the Qatar National Library
Citation: Liaquat Ali. Electrodiagnostic Blink Reflex and Direct Facial Nerve Stimulation; Prognostic Marker in Facial Diplegia. J Neurol Neurophy, 2021, 12(11), 562.
Received: 17-Oct-2021 Published: 17-Nov-2021
Copyright: © 2021 Ali L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.