Research Article - (2020) Volume 11, Issue 7
Introduction: Episodic memory is a complex cognitive process that allows the encoding, storage and retrieval of unique events associated with the context in which they occurred. The memory function is however affected in Parkinson’s disease (PD) and this is quite associated with the functional loss of brain derived neurotrophic factor (BDNF) which is highly expressed within the brain. This study therefore addressed the question of whether prior/post bromelain treatment was able to show an effect on the memory function and BDNF level in 6-OHDA injected rat model of Parkinsonism. Methods: Male Sprague-Dawley rats were injected stereotaxically with the neurotoxin 6- OHDA. The anti-inflammatory agent, bromelain (40 mg/kg i.p) was used to treat a subset of the rats prior to or 24 hr post 6-OHDA lesion. Discrimination index in the neurotoxin injected rats was assessed using the novel object recognition test. The levels of BDNF in the plasma, PFC and hippocampus were measured using enzyme-linked immunosorbent assay. Results: 6-OHDA injection resulted in marked reduction in the discrimination index which was prevented by the treatment with bromelain prior to the lesion. The plasma level of BDNF was increased by the 6-OHDA injection and bromelain treatment, pre- and post 6-OHDA injection decreased the plasma level of BDNF in the neurotoxin injected rats. Meanwhile, a significant increase in cortical BDNF was observed with post-injection bromelain treatment. Conclusion: The plasma level of BDNF increased in the 6-OHDA rat model of PD. BDNF levels in plasma may represent an important early marker of brain damage and memory deficit. Bromelain treatment reversed the neurotoxininduced increase in plasma BDNF levels and memory deficit, suggesting that early treatment with bromelain might be useful in the prevention of memory loss in patients with PD.
BDNF •6-OHDA•Hippocampus•Pre-frontal cortex•Bromelain
Episodic memory is a complex set of human cognitive processes that allow the encoding, storage and intentional recollection (retrieval) of unique events associated with the context in which they occurred [1]. Studies in neurological patients indicate that learning and memory are connected with certain regions of the brain including the prefrontal cortex (PFC) and in particular hippocampus [2,3]. Cognitive impairment in patients with a neurodegenerative disease is common, ranging from mild cognitive impairment to dementia depending on the severity of the neuroinflammation [4].
Learning and memory is dependent on brain derived neurotrophic factor (BDNF) which is highly expressed in the hippocampus [5], thus making it a target for study of the cognitive deficits in neurodegenerative diseases such as Parkinson’s disease (PD). BDNF and other members of the neurotrophin family perform vital functions involving neuron survival, growth and differentiation [6]. Some studies have shown pronounced hippocampal shrinkage accompanied by spatial memory reduction and degeneration of nerve fibres in patients with PD [7,8]. BDNF exerts its effect on survival and function of selected populations of brain dopaminergic, serotoninergic and GABAergic neurons [9]. In addition, nerve regeneration or inhibition of neuronal loss by BDNF was reported to be an effective strategy for PD management [10].
In the periphery, BDNF is found in the plasma and platelets [11] and it is formed by vascular endothelial cells and by peripheral blood mononuclear cells [12]. Despite the size of the protein (27 kDa), BDNF can cross the bloodbrain barrier [13] in both directions from brain to the periphery and from the periphery to the brain [14], via a high capacity saturable transporter system. A positive link between BDNF levels in the brain and serum was described [15].
Consistent with data from animal models, human longitudinal studies have shown that BDNF polymorphisms are associated with neuroinflammation and hippocampal atrophy [16,17]. Following neuroinflammation, expression of the neurotrophin family, including nerve growth factor and other structurally related neuropeptides, such as BDNF, is increased in the hippocampus [18] and is associated with increased risk for memory loss [19]. Also, inflammatoryreactions have been implicated in the progression of many neurological disorders including PD especially when the symptoms are fully out-blown. This further gives an avenue for the anti-inflammatory agents to be considered as treatment in such conditions [20]. In the present study, we aimed to investigate the effect of bromelain as an anti-inflammatory drug on memory deficit in a 6-OHDA rat model of PD and its possible effect on the level of BDNF in the plasma and certain areas of the brain.
For the novel object recognition test, we analysed the time spent with the novel object by the rats in the four groups viz. NN, 6N, Br6 and 24Br (n=10 per group). The effect of 6-OHDA was demonstrated by two-way repeated measures ANOVA as significantly reduced time spent with the novel object (F(1, 36) = 33.86, p < 0.001; 6N post vs. NN post, p < 0.05; Figure.1A. Also, the duration of time spent with the novel object was significantly increased with bromelain pre-treatment of 6-OHDA injected rats (6N post vs. Br6 post), p < 0.05. Similarly, the percentage discrimination index was significantly reduced by 6-OHDA injection F(1, 36) = 7.72, p < 0.001, Figure.1B and was reversed by presurgical bromelain treatment (6N vs. Br6), p < 0.05. The percentage discrimination index was reduced by post-surgical bromelain treatment compared to 6-OHDA injection but not statistically significant.
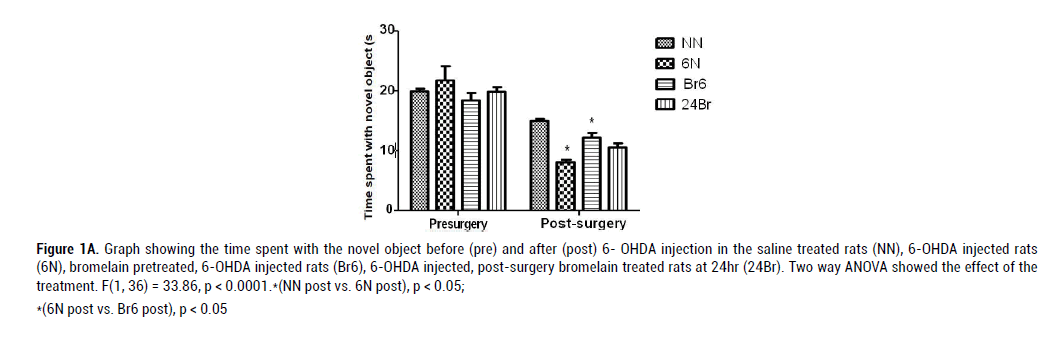
Figure 1A: Graph showing the time spent with the novel object before (pre) and after (post) 6- OHDA injection in the saline treated rats (NN), 6-OHDA injected rats
(6N), bromelain pretreated, 6-OHDA injected rats (Br6), 6-OHDA injected, post-surgery bromelain treated rats at 24hr (24Br). Two way ANOVA showed the effect of the
treatment. F(1, 36) = 33.86, p < 0.0001.*(NN post vs. 6N post), p < 0.05;
*(6N post vs. Br6 post), p < 0.05
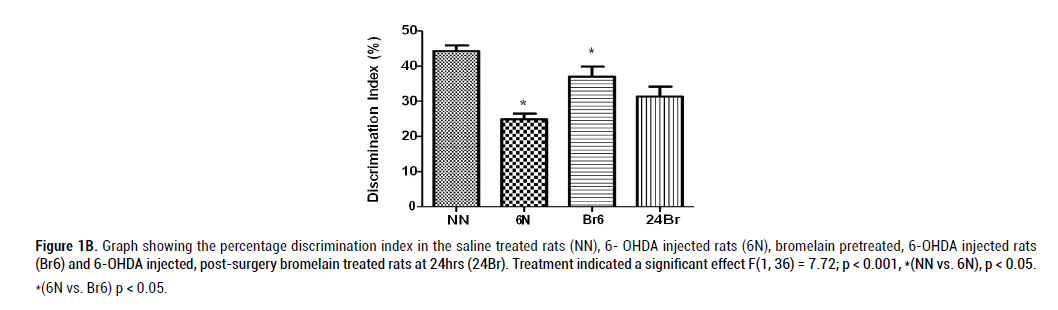
Figure 1B: Graph showing the percentage discrimination index in the saline treated rats (NN), 6- OHDA injected rats (6N), bromelain pretreated, 6-OHDA injected rats
(Br6) and 6-OHDA injected, post-surgery bromelain treated rats at 24hrs (24Br). Treatment indicated a significant effect F(1, 36) = 7.72; p < 0.001, *(NN vs. 6N), p < 0.05.
*(6N vs. Br6) p < 0.05.
The plasma level of BDNF was significantly increased by the neurotoxin (6- OHDA) compared to the control group (NN), F(3, 20) = 5.806, p = 0.0059, Figure. 2A. The systemic BDNF was significantly reduced by bromelain pretreatment (6N vs. Br6), p < 0.05. Also, the post- surgery treatment with bromelain significantly reduced the plasma BDNF level (6N vs. Br6), p < 0.05. There was an effect of treatment on the cortical level of BDNF F(3, 20) = 3.914, p = 0.0259, Figure. 2B. The cortical BDNF level remained unaffected by 6-OHDA injection but was found significantly increased by post-surgery bromelain treatment compared to 6-OHDA injected rats (6N vs. 24Br), p < 0.05. Also, bromelain pretreatment further showed no significant effect on the cortical BDNF level. The injection of 6-OHDA caused an increase in the hypothalamic concentration of BDNF but not significant. The hypothalamic concentration of BDNF was reduced by pretreatment with bromelain but not statistically significant. Also, the hippocampal level of BDNF remained unaffected by the post treatment with bromelain compared to 6-OHDA injection F(3, 20) = 1.691, p = 0.2046, Figure. 2C.
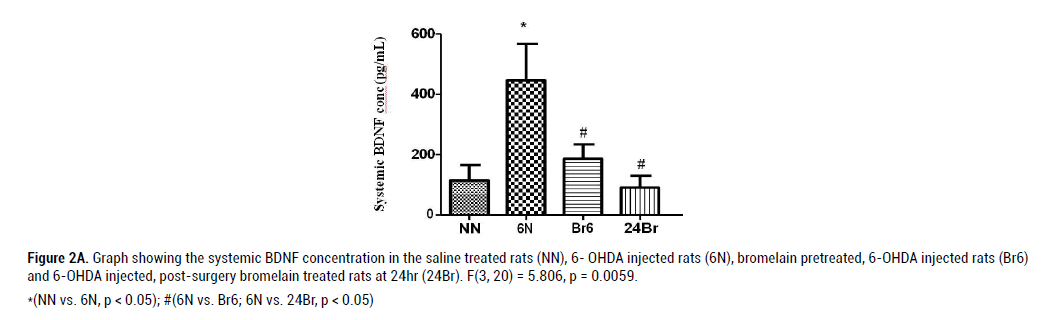
Figure 2A: Graph showing the systemic BDNF concentration in the saline treated rats (NN), 6- OHDA injected rats (6N), bromelain pretreated, 6-OHDA injected rats (Br6)
and 6-OHDA injected, post-surgery bromelain treated rats at 24hr (24Br). F(3, 20) = 5.806, p = 0.0059.
*(NN vs. 6N, p < 0.05); #(6N vs. Br6; 6N vs. 24Br, p < 0.05)
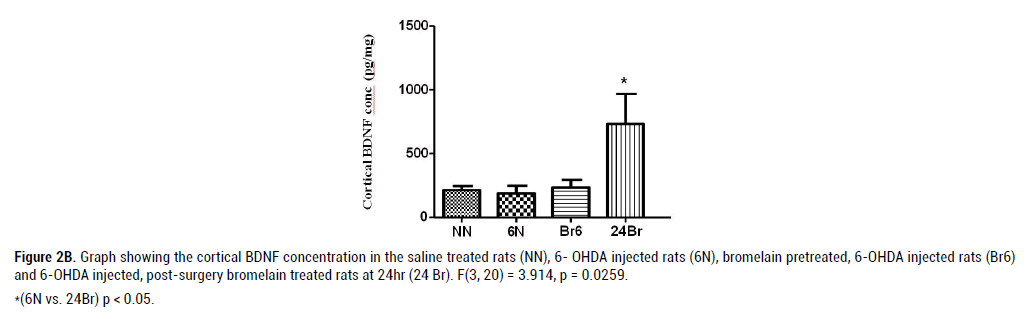
Figure 2B: Graph showing the cortical BDNF concentration in the saline treated rats (NN), 6- OHDA injected rats (6N), bromelain pretreated, 6-OHDA injected rats (Br6)
and 6-OHDA injected, post-surgery bromelain treated rats at 24hr (24 Br). F(3, 20) = 3.914, p = 0.0259.
*(6N vs. 24Br) p < 0.05.
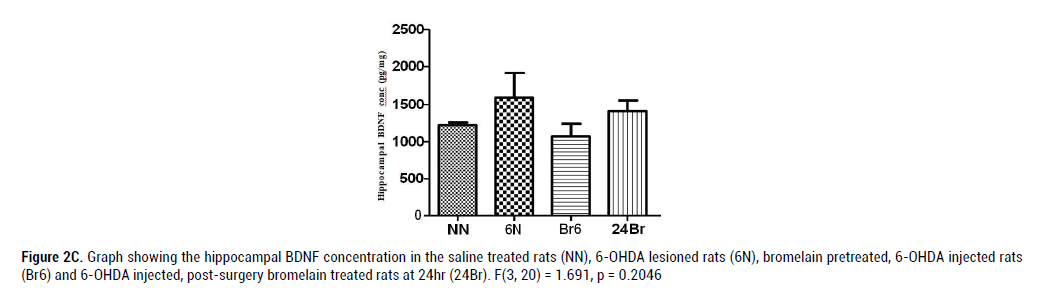
Figure 2C: Graph showing the hippocampal BDNF concentration in the saline treated rats (NN), 6-OHDA lesioned rats (6N), bromelain pretreated, 6-OHDA injected rats (Br6) and 6-OHDA injected, post-surgery bromelain treated rats at 24hr (24Br). F(3, 20) = 1.691, p = 0.2046
The aim of the study was to investigate the changes in memory function in relation to the level of BDNF following 6-OHDA lesion in a rat model of parkinsonism. The impairment of object recognition as an indication of memory dysfunction was observed following the 6- OHDA lesion. Previous studies demonstrated that the neurotoxin could produce the cognitive deficit in animals [21] through the production of hydroxyl radicals during autooxidation [22]. In our laboratory, we were able to show that injection of 10μg of 6-OHDA at the intra medial forebrain bundle resulted into motor impairment as well as depletion in dopamine level both in striatum and PFC [23]. This present study is the continuation of the earlier mentioned research study.
Our results in Figure.1 add to the existing literature by demonstrating that the intra-MFB injection of 6-OHDA caused cognitive impairment by reducing the discrimination index by almost 50%. Bromelain as a treatment drug was administered prior and post intra-MFB 6- OHDA lesion so as to determine the effect of the drug on the impairment incurred by the neurotoxin. In this experiment, there was no effect of post-surgery bromelain treatment on discrimination index compared to 6-OHDA injection but we found that presurgery bromelain treatment improved the discrimination index. This is in agreement with a study reporting that anti-inflammatory treatment improved the cognitive impairment induced by 6-OHDA [24]. It was found that the antiinflammatory drug, quercetin, reversed the cognitive deficit in spatial memory caused by 6-OHDA administration. The mechanism behind this action could be a direct or indirect effect to alleviate the uncontrollable production of cytokines such tumour necrosis factor (TNF-α) and interleukin-1 (IL-1β) [25].
BDNF is a bioactive neurotrophin expressed in the hippocampus and cortex and these functional regions contribute to memory and learning. It is known to be stored in platelets and circulated in plasma but the regulation and function of BDNF in peripheral blood is still poorly understood. In addition, previous studies reported that potential agents of anti-parkinsonism were found to protect against brain lesion via BDNF signal pathway [26,27]. In this study, the level of BDNF was determined in the blood, PFC and hippocampus so as to determine a possible neurochemical basis for the efficacy of bromelain in protecting against memory impairment. The BDNF level in the PFC was increased by bromelain treatment post-surgery. Exercise increased hippocampal BDNF and enhanced the recovery of learning and memory functions aftertraumatic brain injury [28]. This current study showed that bromelain, administered post-surgery, enhanced the level of cortical BDNF. This finding corroborates Mizuno et al., where higher level of cortical BDNF was associated with better working memory performance in animals [29]. Also, the elevated level of BDNF as seen with post-surgery treatment with bromelain may give a further insight into the biological mechanism underlying this drug activity since frontal BDNF is intimately involved with working memory and potent trophic factor for cortical neurons [30].
In PD patients, the serum BDNF was found to decrease but increased afterwards due to longer time span of the disease [31]. Similarly, [32] found that the increase in the level of nerve growth factor is dependent on the extent of neurodegeneration. In our study, the BDNF level was increased in the plasma after 7 days in response to the 6-OHDA lesion. This is in conformity with Saha et al. who showed that alteration in the signaling molecules of BDNF and the tyrosine kinases (TrK) cascade were important events that occurred in response to auto-oxidation of the neurotoxin [33]. This is further corroborated by a previous study reported by Allen et al. (2006) which further implicated activation of BDNF and calcium influx-induced excitotoxic response triggering events leading to cell death in neuropathies such as PD [34]. The elevated plasma level of BDNF is suggested to be a reflection of brain damage. However, the processes causing the increase in the plasma neurotrophic factor after intra- MFB 6-OHDA injection are not known, but the systemic BDNF synthesis as reported by [35] may be attributed to T-cells and monocytes invading the brain. Bromelain treatment reduced the plasma level of BDNF in 6-OHDA injected rats. This actually supplies new information on the plasma BDNF in memory of neurotoxin injected animals. The results obtained confirmed an increase peripheral BDNF level in PD supporting the potential role of neurotrophin in the disease pathogenesis. However, the mechanism by which peripheral BDNF level may reflect the expression in brain is still an open question. Many systemic BDNF sources may synthesize and release the neurotrophin in blood as epithelial cells, vascular endothelial cells and so on [36]. Peripheral levels of BDNF might be linked to the brain neurotrophin content since BDNF might bi- directionally cross the blood brain barrier through an active transport system [37]. Following this report, we therefore suggest that the actions of bromelain in reducing the cognitive deficit may be associated with the restoration of BDNF within the plasma.
Experimental animals and Surgery
All animal experiments were performed according to the NIH guidelines for the care and use of laboratory animals and were approved by the Animal Research Ethics Committee of the University of KwaZulu-Natal (AREC/019/016D). Male Sprague-Dawley rats were housed under a 12 hr light/dark cycle with free access to standard rat chow and water in the Biomedical Resource Unit of the University of KwaZulu-Natal. At PND 51, the animals were divided into two major groups viz pre-surgically treated rats with daily injections of bromelain (40 mg/kg i/p; Sigma-Aldrich, USA), (n = 10) and saline treated animals (10 ml/kg i/p; Adcock- Ingram, SA) (n = 30) for 7 days. The dose of bromelain was based on previous experiments [38]. At PND 60, the animals were injected with desipramine (15 mg/kg i/p; Sigma, Munich, Germany) a norepinephrine reuptake blocker which serves to prevent 6-OHDA uptake by noradrenergic neurons. The rats were deeply anaesthetized with ketamine (90 mg/kg/i.p; Bayer Pty Ltd, SA) and xylazine (5 mg/kg/i.p; Intervet Pty Ltd, SA) was administered to stabilize systemic arterial pressure. The drugs were administered as a concoction and the animals were monitored until they were confirmed to be fully anaesthetized. Following 30 min after the administration of desipramine, the anaesthetized rats were positioned on a stereotaxic frame (Kopf Instruments, Tujunga, USA). The neurotoxin 6-OHDA (10 μg) dissolved in normal saline (4 μL) containing 0.2% ascorbic acid (Sigma, St. Louis, MO, USA) was injected into the left medial forebrain bundle (MFB) using the stereotaxic coordinates AP – 4.7, ML + 1.6, DV – 8.4. Control animals were injected with normal saline (4 μL). Following surgery, the animals were placed on a heating pad until recovery. This was followed by an injection of temgesic (0.05 mg/ kg/s.c; Reckitt Benckiser Ltd, UK) for pain relief. The animals were further subdivided into four groups viz: presurgery saline treatment (10 mL/kg i.p, daily for 7 days) followed by intra-MFB saline injection, with post-surgery saline treatment (10 mL/kg i.p, daily for 7 days; Adcock-Ingram, South Africa) (NN), presurgery saline treatment (10 mL/kg i.p, daily for 7 days) followed by intra- MFB 6-OHDA injection, with post-surgery saline treatment (10 mL/kg i.p, daily for 7 days) (6N), presurgery bromelain treatment (40 mg/kg i.p, daily for 7 days; Sigma-Aldrich, USA) followed by intra-MFB 6-OHDA injection with post-surgery saline treatment (10 mL/kg i.p, daily for 7 days) (Br6) and presurgery saline treatment (10 mL/kg i.p, daily for 7 days) followed by intra-MFB 6-OHDA injection, with daily post-surgery bromelain treatment starting from 24 hr after surgery for 7days (24Br) (Table 1). Behavioural assessment took place after the last drug/vehicle injection.
| Groups | Pre-surgery Bromelain Treatment | 6-OHDA (10μg/4μL) Lesion | Post-surgery Bromelain Treatment |
|---|---|---|---|
| NN | - | - | - |
| 6N | - | + | - |
| Br6 | + | + | - |
| 24Br | - | + | + |
Table 1: Treatment schedule of the four groups
Behavioural Assessment
On PND 58, two days before surgery, memory was tested in all the rats using the novel object recognition test. The same test was repeated after the last post-surgical treatment with bromelain or saline.
Novel Object Recognition test
Each animal was individually placed in a square open arena (68× 68× 45) cm3 for three sessions, each of 10 min duration so as to habituate the animal to the apparatus and the test room. In the object recognition task, each rat was first placed in the box and exposed to two identical sample stimuli (A1 and A2) for 5 min. These objects were placed close to two adjacent corners. The rat was then returned to its cage. During the retention interval, the experimenter removed both objects and replaced one by its identical copy (A) and the other one by a new object (B). After a delay of 24 hr, the rat was placed back in the box and now exposed to the familiar object (A, object identical to A1 and A2) and a novel test object (B, new object) for a further 5 min in the same locations as the previous objects. The total time spent exploring the two objects in both the sample and test periods were recorded. Exploration was operationally defined as directly attending to the object with the head no more than 2 cm from the object [39]. New objects were used for every session. The first phase of the object location test was exactly the same as the recognition test. The discrimination index (DI) was expressed as the difference between the exploration time for the novel object and that of the familiar object divided by the total exploration time. The DI was expressed in percentage to measure the recognition memory [40].
Animal Sacrifice and tissue collection
A subset of the animals (6 per group) was randomly selected and sacrificed by decapitation 24 hr after the last treatment with either bromelain or saline. The blood was collected in separate heparinized tubes and centrifuged for 15 min at 1160×g in a 4ºC refrigerated centrifuge (HERMLE LABTECH, Germany) after which plasma was pipetted into eppendorff tubes. The brain was also removed immediately after decapitation and placed in a frozen 0.9% saline slush so as to suppress the degradation of brain structures during dissection. The PFC and hippocampus were dissected out, weighed and placed in eppendorff tubes. Then, the plasma, PFC and hippocampal tissues which were already placed in eppendorff tubes were further snap-frozen in liquid nitrogen before being stored in a -80ºC bio-freezer until further use. The remaining animals (4 per group) were also decapitated. The brains were removed and stored in the -80ºC bio-freezer for further research purposes.
Determination of BDNF levels in plasma, PFC and hippocampus
In order to gain insight into the neurochemical basis for memory dysfunction, the BDNF levels in the plasma, PFC and hippocampus were analysed using a Sandwich-ELISA method (Elabscience Biotech. Texas, USA). The analysis protocol for BDNF consisted of both extraction and quantification procedures. Both steps were conducted on the same day. The micro ELISA plate provided with the kit was already pre-coated with antibody specific to BDNF only in the samples. The PFC and hippocampal tissues were removed from the biofreezer and the tissues were immediately minced in ice cold PBS (1 mg of tissue/4 mL) and further homogenized in a sonicator (CML-4, Fischer, USA) before being centrifuged at 1160×g for 10 min at 4ºC. The supernatant was pipetted into new eppendorff tubes. The standard, control and the tissue samples (PFC, hippocampus and plasma) were respectively pipetted into each well of the respective micro ELISA plate. The biotinylated detection antibody specific to BDNF (100 μL) was added to each well and incubated for 1 hr at room temperature. Following this, was the addition of avidin conjugated horseradish peroxidase (HRP) (100 μL) to each well and incubation for 30 min at room temperature. This was then followed by the addition of 3,3ˈ,5,5ˈ- tetramethylbenzidine (TMB) substrate (90 μL) to each well. The microplate was then incubated again at room temperature for 15 min. Following this incubation, the corresponding stop solution comprising sulphuric acid (50 μL) was added. The absorbance of the BDNF was quantified using a microtitre plate reader (SPECTROstar Nano, BMG LABTECH GmbH, Ortenberg, Germany) at a wavelength of 450 nm ± 2 nm within 10 min as per the manufacturer’s protocol. All samples, standard and control were analysed in triplicate. The assay guidelines provided by the manufacturer were followed (Catalogue No: E-EL-R001).
Statistical Analysis
All results were presented as mean ± SEM. The data were analysed using GraphPad Prism (version 5, San Diego, California, USA). Data normality was assessed by the Kolmogorov- Smirnov tests. The behavioural outcome was compared using two-way repeated measure of analysis of variance (ANOVA) followed by Bonferroni post hoc analysis while other outcomes were compared with 6-OHDA injected rats using one way ANOVA. Effects were considered statistically significant when p value < 0.05.
In conclusion, our study showed a significant increase in the plasma level of BDNF in the 6- OHDA rat model of PD. Bromelain treatment reversed the increase in BDNF levels in the plasma. Therefore, BDNF levels in plasma may represent an important early marker of brain damage and it may be hypothesized that bromelain could play a vital role in the memory deficit during PD with considerable readjustment in the level of BDNF.
The authors hereby declare there is no conflict of interests associated with this study or any of the procedures and materials used for the purpose of the study.
Citation: Temitope SA&Musa VM. Effect of Bromelain on BDNF level and memory deficit following intra-medial forebrain bundle 6-OHDA injection in rat model of Parkinsonism. J NeurolNeurophy, 2020, 11(7), 508.
Received: 28-Oct-2020 Published: 25-Nov-2020, DOI: 10.35248/2155-9562.20.11.504
Copyright: 2020 Temitope SA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.