Journal of Multiple Sclerosis
ISSN - 2376-0389NLM - 101654564
Case Report - (2023) Volume 10, Issue 6
Tuberous Sclerosis Complex (TSC) is a genetic neurocutaneous disorder that presents with multi-organ involvement, including hamartomas in the brain, eyes, heart, lung, liver, kidney and skin. Multiple Sclerosis (MS) is a complex chronic inflammatory and neurodegenerative demyelinating disease of the Central Nervous System (CNS) of unknown etiology.
We report a 37-year-old woman with TSC who developed gradual tingling and hypoesthesia in her left hemi some. Magnetic resonance imaging (MRI) of the brain and cervical spine showed multiple cortical and subcortical tubers and sub ependymal nodules, along with diffuse and periventricular T2 white matter changes, and T2-STIR hyper intensity with edema at C3 level without gadolinium enhancement. Blood test was positive for multiple auto-antibodies not matching any specific autoimmune disease and lumbar puncture evidenced Oligo Clonal Bands (OCB) restricted to the cerebrospinal fluid, pattern II. Considering the OCB detection, the presence of spinal and supra-tentorial demyelinating lesions and the clinical myelitis, a diagnosis of an autoimmune disease of the CNS, MS, was made. She was started on Teriflunomide as maintenance therapy. She continued with her regular follow-up and one year later showed no new clinical or radiological relapse.
A common pathogenic mechanism can be suggested in the present case as dysregulation of the Mammalian Target of Rapamycin (mTOR) pathway is implicated in TSC pathology and in immuno-modulatory mechanisms.
Tuberous Sclerosis Complex • Oligo Clonal Bands • Magnetic Resonance Imaging
We report a 37 year-old woman with Tuberous Sclerosis Complex (TSC) who was hospitalized in June 2022 after the development of tingling and hypoesthesia in the left arm, trunk and leg. She was completely asymptomatic for TSC which was previously diagnosed following a Magnetic Resonance Imaging (MRI) performed for nonspecific tremors. The MRI evidenced sub ependymal nodules and cortical dysplasia with tubers and white matter migration lines 1, no other organ was involved. She underwent genetic testing which detected TSC1 mutation (c.1641_1642delAC) and mosaicism and started brain MRI monitoring every 3 years.
She also presented cutaneous leuko-cytoclastic vasculitis in 2002 and erythema nodosum in 2007; both treated with steroids with complete remission. Her neurological symptoms started in May 2022 with progressive onset of left brachio-crural tingling and hypoesthesia associated with reduced manual dexterity. She was treated with intramuscular betamethasone 9mg/day for 3 days with gradual symptoms resolution. Brain MRI with gadolinium showed multiple cortical and subcortical tubers, sub ependymal nodules and white matter T2 hyper intensities described as migration lines and periventricular lesions; cervical MRI evidenced an area of T2-STIR hyper intensity with edema located at C3 level in the left dorsolateral columns without gadolinium enhancement (Figure 1).
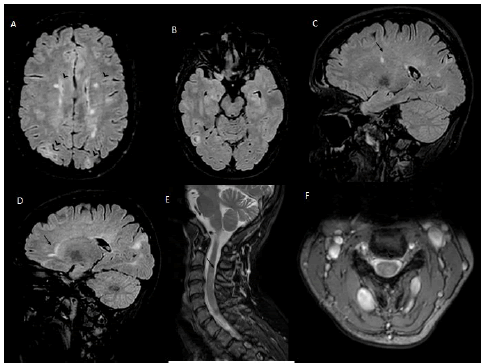
Figure 1: Axial T2 FLAIR MRI showing (A,B) cortical, subcortical and periventricular hyper-intensities within the brain parenchyma (arrowhead); (C,D) Sagittal T2 FLAIR MRI showing periventricular (arrow) and typical cortical/subcortical hyper-intensities and white matter migration lines (arrowhead). (E,F) Sagittal and axial T2 MRI of the spine showing an area of hyper-intensity at C3 level (arrow). FLAIR Fluid Attenuated Inversion Recovery; MRI Magnetic resonance imaging.
She was therefore hospitalized for diagnostic evaluation. She reported no recent infection, oral-ocular dryness, arthralgia, fever, oralgenital ulcers, dyspnea, photosensitivity or Raynaud’s phenomenon. Neurological examination only showed mild left brachio-crural hypoesthesia and a Lhermitte sign. Differential diagnoses included Aquaporin-4 Antibodies positive Neuromyelitis Optica Spectrum Disorder (NMOSD), Myelin Oligodendrocyte Glycoprotein (MOG) antibodyassociated disease (MOGAD); secondary causes of myelitis: infectious, vitamin deficiency, and autoimmune, such as Sjogren syndrome, Systematic Erythematous Lupus (SLE) and sarcoidosis.
Blood test was significant for positive several autoantibodies: antinuclear antibodies, extractable Nuclear Antigen (anti-Sjogren syndrome type A Ro52/Ro60, type B; anti-Smith) Anti- cyclic citrullinated peptide, Anticardiolipin IgG, ant neutrophil cytoplasmic antibodies (c-ANCA) and perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA). MOG and Aquaporin (AQP4) IgG antibodies were absent and chest X-ray was normal. Serum infectious panel (hepatitis B and C virus, cytomegalovirus, human-immunodeficiency virus, varicella-zoster virus, Borreliosis, Quantiferon) was unremarkable. Cerebrospinal fluid (CSF) biochemical analysis showed mild lymphocytic pleocytosis with normal protein and no blood-brain-barrier damage. Isoelectric focusing electrophoresis evidenced 9 Oligo Clonal Bands (OCB) restricted to the CSF.
Brain scans were therefore re-evaluated and few supra-tentorial T2 hyperintensities were re-classified as being periventricular/demyelinating Dawson’s fingers like and not due to TSC. In light of the presence of CSF restricted OCB, multiple autoimmune antibodies positivity not matching any specific autoimmune disease, the presence of spinal and supra-tentorial demyelinating lesions, the clinical myelitis and a no better explanation, a diagnosis of Relapsing-Remitting (RRMS) with associated autoantibodies was made. The patient was then started on Teriflunomide with good compliance. She continued with her regular follow-up, with no evidence of disease activity at 12 months follow-up.
Brain MRI findings in TSC are abundant with a wide spectrum of possible manifestations such as focal dysplasia, hamartomas, sub ependymal nodules and widespread gray and white matter signal changes, nevertheless spinal cord abnormalities are rarely seen [1]. In our patient the cervical myelitis in accordance with OCB pattern II detection led to the diagnosis of RRMS. Although an association between MS and other autoimmune diseases has been described, its concurrence with TSC is less known [2].
There are few reports describing a coexistence of TSC with SLE and with AQP4 positive NMOSD [3-6]. The association between TSC and autoimmune diseases or abnormal production of autoantibodies could have a common pathogenesis. In TSC, mutations of the TSC 1 and TSC 2 genes leads to an abnormally activated mammalian target of rapamycin pathway, which is one of the main pathways regulating development, differentiation, metabolism, and function of T and B cell.
Subsequently, dysregulation of B and T lymphocytes may cause abnormal responses with an increased risk of autoimmunity [7]. In support of the latter our patient presented in her medical history an episode of erythema nodosum, which is linked to immune complex deposition, and eukocytoclastic vasculitis, which can be found in various forms of autoimmune vasculitis and associated to systemic diseases such as SLE [8,9]. SLE, which has already been associated with TSC 3–5, is characterized by a florid autoantibody production and can also cause myelitis.
A diagnosis of SLE was excluded in our patient because brain MRI did not show calcifications, hemorrhages and infarcts that are frequently present in SLE. Moreover, compared to MS, the incidence of myelitis in SLE is more rare and usually more severe with longitudinal extensive involvement and ischemic necrosis and vasculitis, which, also considering the good outcome, was allegedly not the case of our patient [10]
Taking into account the MRI, clinical and laboratory data and a no better explanation, we diagnosed RRMS. Reporting such cases is important for expanding our understanding of the interplay between genetics and autoimmunity and determining whether TSC might confer a higher risk to develop autoimmune diseases such as MS.
Citation: Bruschi, N., et al. Does the Coexistence of Tuberous Sclerosis and of Multiple Sclerosis in a Young Woman have a Common Pathogenic Mechanism? J Mult Scler. 2023,10, (06), 501.
Received: 12-Jun-2023, Manuscript No. jmso-23-102193; Editor assigned: 14-Jun-2023, Pre QC No. jmso-23-102193 (PQ); Reviewed: 25-Jun-2023, QC No. jmso-23-102193 (Q); Revised: 27-Jun-2023, Manuscript No. jmso-23-102193 (R); Published: 30-Jun-2023, DOI: 10.35248/2376-0389.23.10.06.501
Copyright: ©2023 Bruschi N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.