Research - (2023) Volume 14, Issue 3
Background: Eye is a biologic camera gifted to us and ocular movement is a very easy task but provides useful information regarding the diagnosis of parkinsonian disorders. Some interpretation of the abnormalities are highly characteristic of a specific disease i.e., progressive supranuclear palsy, oculogyric crisis in post encephalitic Parkinson’s but many are nonspecific .symptoms of parkinsonism regarding the eye are nonspecific. Retina contains dopamine, hence the ocular symptoms like astenopia are common in PD. This study has been done with a purpose of providing an overview of eye movement abnormalities in Parkinsonism.
Materials and methods: 100 patients attending neurology OPD diagnosed as Parkinson’s disease and Parkinson plus syndromes. Demographic details, detailed history, ocular signs and symptoms, ocular deviation, visual acuity, diagnosis, and management aspects were extracted from the medical records. Visual acuity by Snellen values. Ocular deviation was measured by prism cover test, and ocular motility was carried out to evaluate the gaze limitation. A diagnosis of CI was made if Near Point of Convergence (NPC) value was larger than 10 cm. Saccade and pursuit scores evaluated using the Northeastern State University College of Optometry method was used to determine if they were lower than the age expected norms. Color vision was assessed using Ishihara pseudo isochromatic plates. Reading speed was measured in Words Per Minute (wpm) based on reading a simple paragraph in an English magazine for a minute. Developmental Eye Movement (DEM) rate was calculated based on the time taken for the vertical and horizontal tasks on the DEM test. Protocol of eye movement examination was followed
Conclusions: Parkinsonian group of disorders are a unique group that in most of the cases eye movement gets affected and we get a valuable clue for the diagnosis. The most frequently affected include saccades, smooth pursuit and fixation. Asthenopia-Decreased vision associated with reading difficulty was common in PD patients. In terms of gaze restriction, vertical gaze involvement) was more than horizontal involvement Convergence Insufficiency (CI) was the most common binocular vision dysfunction ,followed by CI with oculomotor dysfunction and vertical gaze palsy. Ground prisms were recommended for few patients and home vision therapy for few patients as corrective measures.
Parkinsonism • Anhedonia • Progressive Supranuclear Palsy (PSP) • Niemann-Pick Type C (NPC) • Spinocerebellar Ataxia Type 2 (SCA2) • Opsoclonusmyoclonus ataxia syndrome (OMAS) • Multiple System Atrophy (MSA) • Rostral Interstitial Nucleus of the Medial Longitudial Fasciculus (RIMLF) • Interstitial Nucleus of Cajal • (INC) • Paramedian Pontine Reticular Formation • (PPRF) • Nucleus Raphe Interpositus • Medial Vestibular Nucleus/Nucleus Prepositus Hypoglossi (MVN/NPH)
Background
The extrapyramidal system is more of a functional concept .It can be looked at as a neural network that influences motor control. The basal ganglia contribute most extensively to the extrapyramidal system .The basal ganglia In addition to motor circuits controlling voluntary movement have limbic connections involved with the emotional aspects of movement as well as connections with the oculomotor system. With the connections of oculomotor system they modulate the eye movements which are the objective of the study [1, 2].
• The horizontal saccades excitatory burst neurons are located in the Para Median Pontine Reticular Formation (PPRF) in the pons [3,4].
• The Medial Vestibular Nucleus/Nucleus Prepositus Hypoglossi (MVN/NPH) in the medulla are the horizontal neural integrators.
• For vertical saccades, excitatory burst neurons are predominantly located in the rostral interstitial nucleus of the Medial Longitudinal Fasciculus (RIMLF), and the Interstitial Nucleus of Cajal (INC) is the vertical neural integrator. Both of these are in the midbrain.
• The nucleus Raphe Interpositus (RIP) in the pons houses the Omni pause neurons. Lesion may not be direct lesion of the MVN/NPH, but may be lesion of the cerebellar feedback circuitry to these structures [5]
Major loops of the basal ganglia: Direct loop is excitatory and the indirect loop is inhibitory. As an overview, direct pathway is mediated by D1 receptors and results in facilitation of movement [6]. The indirect pathway is mediated by D2 receptors and results in inhibition of movement. In the direct pathway the motor cortex sends glutamatergic activating signals to the caudate and putamen and excites D1 receptors. Striato pallidal fibres to the Globus pallidus internal and superficial zona reticulate are inhibitory. Fibers from the GP I to the thalamus are inhibitory. At rest GPi /SNr exert in inhibitory influence on the thalamus on the cortex [7].
Activation of the direct pathway inhibits the activity of GPi/SNr. The direct loop projections from the striatum to the GPi inhibit the inhibitory pallid thalamic pathway and result in net cortical excitation and facilitation. The direct loop excites the thalamic projection to the cortex by inhibiting the pallid thalamic pathway [8].
Indirect Pathway
The motor cortex sends glutamatergic activating signals to the caudate and putamen and excites D2 receptors [9]. Striatopallidal fibers then project to the GPexterna, causing inhibition. The resting activity of the GPe decreases. The GPe projects inhibitory GABAergic fibers to the sub thalamic nucleus by the sub thalamic fasciculus [10]. The sub thalamic nucleus ten projects to the GPi, but its fibers are excitatory (glutamatergic).
Indirect pathway activity reduces the inhibition of the STN. The STN then facilitates the inhibitory projections of the GPi to the thalamus, resulting in a net decrease in activity in the thalamocortical pathways. The indirect loop inhibits the thalamic projections to the cortex by increasing the inhibition mediated by the pallid thalamic projection. Activity in the indirect loop prevents activation of motor cortical areas that might interfere with the voluntary movement executed by the direct pathway [11]. Activity in the direct loop results in the net increase in cortical excitation. Activity in the indirect loop results in a net decrease as shown in Figures 1-4.
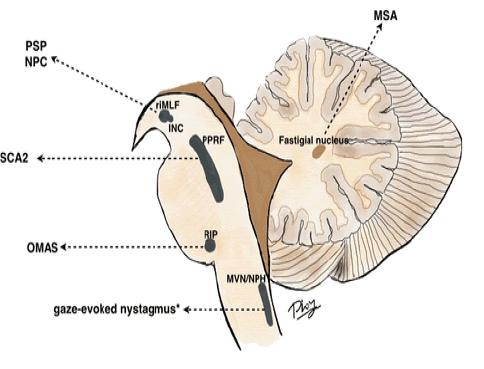
Figure 1: This figure illustrates the brainstem excitatory burst neurons and neural integrators for horizontal and vertical saccades, and examples of disorders affecting these structures.
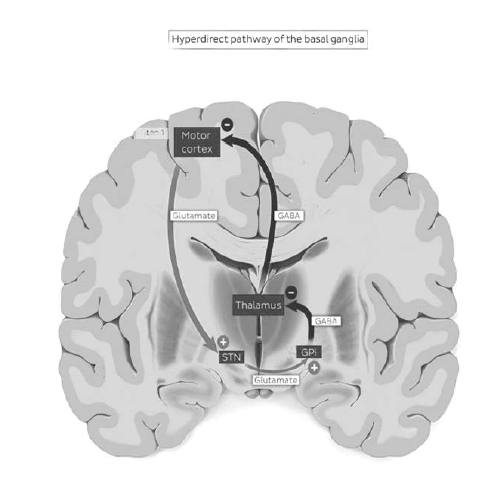
Figure 2: Hyperdirect pathway of the basal ganglia.
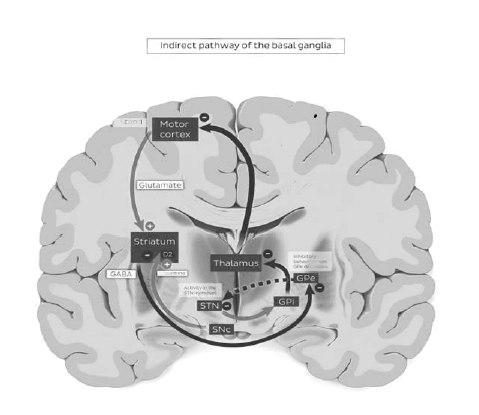
Figure 3: Indirect pathway of the basal ganglia
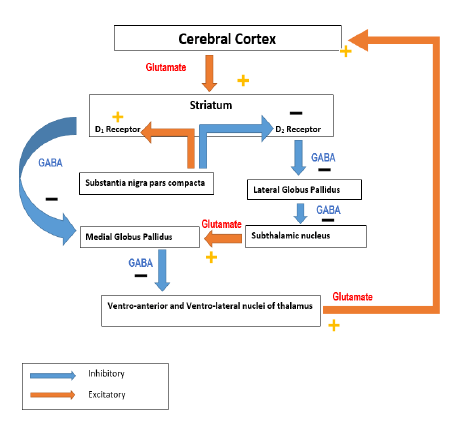
Figure 4:There are multiple types of eye movements including smooth pursuit, saccades, vestibular and optokinetic reflexes, and vergence.
Saccadic Pathway
In the absence of saccade activity, the excitatory burst neurons are inhibited by Omni pause neurons. Initiation of the saccadic pulse occurs when the burst neuron is released from its tonic inhibition and initiation of saccades, pulse command is generated by firing of excitatory burst neurons, which are the Para Median Pontine Reticular Formation (PPRF) in the pons and the Rostral Interstitial Nucleus of the Medial Longitudinal Fasciculus (RIMLF) in the midbrain, for the horizontal and vertical saccades, respectively (Figures 5, 6) . When the eyes reach the new position, step command keeps the eyes with the new level of tonic firing of the responsible neurons, which are the Medial Vestibular Nucleus (MVN) and Nucleus Prepositus Hypoglossi (NPH) in the medulla and the Interstitial Nucleus of Cajal (INC), for the horizontal and vertical saccades, respectively [12].
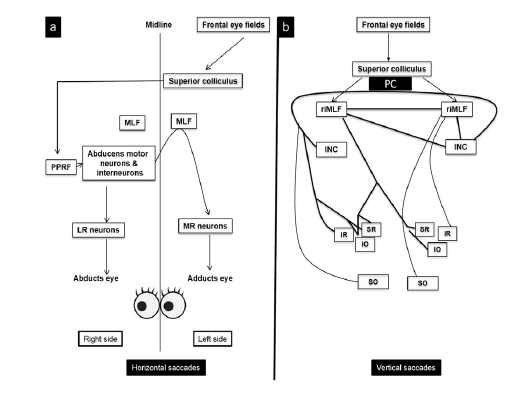
Figure 5: Rapid changes in orbital eye position are by saccadic system at a peak velocity of 400 degree/second. Described according to latency, amplitude, velocity and gain.
Figure 6: SACCADES: Initiation of a saccade requires a â??pulseâ? of increased firing of excitatory burst neurons in the brainstem that results in a high-frequency burst of phasic activity in agonist extraocular muscles.
Higher level structures including the frontal and parietal lobes, as well as the substantia nigra reticulata and superior colliculi also play critical roles in saccade generation. Most rapid changes in orbital eye position are achieved using saccades at velocity of 400 degree/second.
Voluntary saccades are tested by asking the patient to look at both sides. Visually reflex saccades tested by presenting a visual stimulus in one half of the visual field consequently. Auditory reflex saccades tested by asking the asking the patient to look to a sound on either sides. Suddenly suppressing visual look to one hemi field shifting vision in the presence of stimulus being presented in another field [13].
Predominant saccades
Spontaneous saccades internally triggered without a goal occur during other motor activities: speech, rest, sleep [14]. Excessive generation of spontaneous saccades occur with impact visual fixation and with opsoclonus. Reflexive saccades are externally driven by the sudden appearance of a visual target on the peripheral retina or a sudden noise. Volitional saccades are internally triggered and have a goal or intentional purpose but they are highly susceptible to external modification by the demands of the stimulus [15].
Pursuit
We are capable of pursuing slowly moving visual objects with their eyes. The aim of these Smooth Pursuit Eye Movements (SPEMs) is to minimize the error between the image of an object of interest and the retinal area of high acuity vision, the fovea There are at least two cortical areas that crucially contribute to smooth pursuit and are therefore eligible sites for dynamic gain control: the Medial Superior Temporal area (MST) and the pursuit area of the Frontal Eye Fields (FEFs), which both project to brain stem premotor structures via parallel pathways. It is a controlled feedback system because the motor output (eye movement) alters the sensory input [16].
The pursuit system is a controlled feedback system because the motor output (eye movement) alters the sensory input (retinal image). A consequence of this closed-loop control scheme is that the retinal error by itself is not sufficient to drive the system because its value approaches zero during successful SPEM. Several models have been proposed that utilize different signals to maintain the drive for the pursuit system: for example, position and acceleration of the target perceived target velocity based on an efference copy of the motor command sent to the eye muscles, or a velocity storage mechanism implemented as a local cortical feedback loop [17].
Dynamic gain control
A special property of the smooth pursuit system is the dynamic gain control mechanism. This gain control regulates the feedforward gain of the pursuit system in dependence on the actual tracking velocity, where higher tracking velocities yield higher gains. Implicit evidence for such a mechanism was first given by, where spontaneous oscillations were observed during pursuit, but not during fixation of a stationary target. Observed a difference in the onset and offset of SPEM and concluded that different systems contribute to fixation and pursuit (fixation is not pursuit at velocity zero [18]. then measured the ocular responses to constantly moving targets in macaque monkeys with a perturbation in target trajectory, i.e., one cycle of a highfrequency sinusoidal oscillation. Monkeys showed an increased eye velocity response with respect to the perturbation that depended monotonically on the underlying constant speed of target and tracking motion. Gain control also occurs in human smooth pursuit eye movements as has been revealed by similar perturbation experiments used a somewhat different approach, using a continuous high-frequency hum superimposed on the constantvelocity target to test the gain of the system in macaque monkeys and developed a conceptual model of a gain control mechanism in smooth pursuit [19].
Acceleration saturation
Another nonlinear effect that governs smooth pursuit behavior is the saturation of maximum eye acceleration during smooth pursuit initiation, also termed the main-sequence of smooth pursuit [20]. During pursuit of step-ramp targets at different velocities, this results in an increasing latency of the first eye velocity overshoot during SPEM onset for increasing target velocities. At first glance, this is contradictory to dynamic gain control, which increases the gain and therefore reduces the latencies at higher tracking velocities. However, both effects have been reported independently of each other and no model has dealt with the simultaneous occurrence of both nonlinear effects [21].
Cortical contribution to smooth pursuit
Neural signals related to the motion of an object’s image on the retina are found in the Middle Temporal area (MT) of the superior temporal sulcus in primates. These cells constitute the primary motion processing stage, receiving afferent signals from the dorsal pathway via the retina, Lateral Geniculate Nucleus (LGN), and from directionally selective cells of primary visual cortex (V1) 25 MT neurons are selective for the direction and the speed of motion and can be physiologically and anatomically subdivided into cells that are responsive to small-sized moving stimuli and cells responding only in the presence of a large moving field [22]. MT sends strong projections to the medial superior temporal area where a similar differentiation can be observed. Cells in the lateral aspect of MST prefer small-sized moving stimuli, whereas cells in the dorsal part (MSTd) respond best to large-field stimuli. Furthermore, cells in MSTl convey extra retinal signals related to slow eye and head movements and might therefore play a role in estimating the target movement in world-centered coordinates. Signals related to slow eye movements are also found in MSTd, but their discharge follows the onset of the eye movement 50 ms to ≥ 100 ms. The activity is maintained during SPEM despite blinking or stabilization of the target and may thus constitute an efference copy of the eye motor command. The preference for large-field stimuli and the additional extraretinal components make the MSTd neurons an eligible candidate for the compensation of self-induced visual motion [23].
Another cortical area that is crucially involved in SPEM is the Frontal Eye Field (FEF), which is also involved in the generation of saccadic eye movements [24]. The first investigators to report the importance of the FEF in SPEM were and. FEF has strong reciprocal connections with MST and lesions therein abolish predictive and visually guided SPEM [25]. The majority of FEF neurons discharge before the onset of pursuit and thus contribute to the initiation of pursuit, which is characterized by high retinal slip and eye acceleration [26].
Recently, the role of the thalamus in the control of SPEM has been reinvestigated. Neurons in the central thalamus carry eye velocity related signals that are conveyed to cortical areas, such as FEF. Conversely, the central thalamus receives inputs from subcortical pursuit pathways, including the vestibular nuclei and the deep cerebellar nuclei. This thalamocortical eye velocity feedback may be used for the control and monitoring of eye movements during ongoing smooth pursuit [27].
Common ocular signs in parkinsonian disorders
Distinctive features of PSP are the presence of ocular signs, which can help in diagnosing the disease [28, 29].
These ocular signs include:
• Supranuclear vertical gaze palsy: This is a hallmark feature of PSP. The patient may have difficulty in tracking moving objects, and this can result in falls and other accidents.
• Square wave jerks: These are involuntary eye movements that involve a brief, rapid deviation of the eyes away from the target, followed by a return to fixation. They can occur in any direction of gaze, but they are most commonly seen when the patient is looking straight ahead.
• Slow saccades: Patients with PSP may have difficulty in making rapid, voluntary eye movements, such as when shifting gaze from one object to another.
• Eyelid apraxia: This is a condition in which the patient has difficulty in opening and closing the eyelids voluntarily
• Blurred vision: Patients with PSP may experience blurred vision, which can make it difficult to read or perform other visual tasks.
The ocular signs in PSP can be quite characteristic, and they can be useful in distinguishing PSP from other neurodegenerative disorders. Ocular symptoms are not typically considered a hallmark of CBD, some patients with the disease may experience visual disturbances, such as difficulty with visual processing, blurred vision, or double vision. Some of the most common ocular signs in MSA include:
• Diplopia: Double vision can occur in MSA due to weakness or paralysis of the muscles that control eye movements.
• Ptosis: Drooping of the eyelids (ptosis) can occur in MSA due to weakness of the levator palpebral muscles.
• Saccadic eye movement abnormalities: MSA can cause abnormalities in the rapid eye movements (saccades), leading to difficulty with eye movements such as difficulty with rapid sideto-side movements.
• Blepharospasm: MSA can cause involuntary spasms of the eyelid muscles, leading to difficulty opening or closing the eyes.
• Reduced blink rate: MSA can cause a reduction in the rate of blinking, leading to dry eyes and other ocular symptoms [30].
Protocol for eye movement examination:
• Extent of range of ocular movement in horizontal and vertical plane
• Fixation stability in central and eccentric gaze-look for nystagmus or saccades that intrude on fixation.
• Horizontal and vertical saccades
• Horizontal and vertical moving target
• Optokinetic nystagmus
• Ocular alignment during fixation of a distant target and vergence responses to smooth or stepping motion of a target aligned in the patient’s saggital plane
• The vestibular ocular reflex.
Nsuco oculomotor test
Purpose: To assess the quality of both saccadic and pursuit eye movements (Table 1).
Table 1. Inclusion and Exclusion Criteria’s
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
| Diagnosis of PD according to UKPDSBB criteria | Hoehn and Yahr Parkinson’s staging score > 4 |
| The patient must be able and willing to give written | Secondary cause of parkinsonism as detected by history( e.g. drug induced parkinsonism) |
| Informed consent | |
| The patient must be willing to participate in all study related activities and visits | Secondary cause of parkinsonism as detected by investigation( e.g. vascular parkinsonism as detected by neuroimaging) |
| Age of onset of Parkinson’s disease > 30 years | Dementia according to DSM-IV |
| Stable doses of Parkinson’s medicine > 4 weeks | Major depressive disorder according to DSM-IV |
| Current age > 60 years | Psychotic disorder(s) according to DSM-IV |
| Prior brain surgery ( except deep brain simulation) | |
| Previous eye surgery(except phacoemulsification for cataract and artificial lenses) | |
| Blindness in 1 eye | |
| Medication that influences normal visual function other than PD medication | |
| Systemic diseases that may cause eye problems ( HIV,DM type I, type II if the patient had ophthalmologic therapy and / or abnormalities at last screening) | |
| Neurodegenerative diseases other than Parkinson’s disease | |
| History of lesions near the optic chiasm or occipital cortex | |
| Migraine | |
Indications: Allows clinician to provide a quick, inexpensive analysis of the patient’s eye movements with minimal patient cooperation
Apparatus and Setup: Two small (approximately 1/2 cm in diameter) coloured, reflective spheres (balls) mounted on dowel sticks. One target is used for pursuits, two for saccades. For those unwilling or unable to be tested with the coloured ball targets, use animated toy targets on pencils (one for pursuits, two for saccades).
Time Required: Minimal time is required, less than two minutes. In an effort to maintain test validity and reliability, the following administration and scoring instructions are adapted directly from the test manual for the NSUCO Oculomotor Test. Proper use of the test and norming of results requires the purchase of the NSUCO Oculomotor Test and adherence to the protocol listed in the test manual.
Pre-set (Administration): The protocol for proper administration is given as shown in the NSUCO Oculomotor Test manual written by W. C. Maples, O.D. I.
Posture: The patient stands with feet a shoulder width apart, arms hanging naturally at their side, directly in front of the examiner. II. Head: Do not give instructions on head movement. Scoring of the test is based on whether or not the patient chooses to use their head for assistance.
Target characteristics: As seen above in "Apparatus and Setup".
Movement of the target: A. Directional:
• Saccades are performed in the horizontal meridian only (five round trips). 2. Pursuits are performed rotationally, both clockwise (two rotations) and counter clockwise (two rotations).
B. Extent:
• Saccade extent should be no more than 10 cm on each side of the patient's mid-line (20 cm total).
• Pursuit path should be no more than 20 cm in diameter. The upper and lower extent of the circular path should coincide with the patient's mid-line.
Test distance from the patient: Testing is done no more than 40 cm and no less than the Harmon distance, i.e., the distance from the subject's middle knuckle to his elbow
Ocular condition: Testing is done binocularly
Age of the patient: The manual states that the test can be performed on anyone two years old to adult, however, the norms are calculated for 5 years old to 14 years old and The manual states that the test can be performed on anyone two years old to adult, however, the norms are calculated for 5 years old to 14 years old and above.
Instructions:
• Saccades: "When I say red, look at the red ball (dog toy). When I say green, look at the green ball (dinosaur toy). Remember, don't look until I tell you to."
• Pursuits: "Watch the ball (dog) as it goes around. Try to see yourself in the ball (watch the dog's eyes). Don't ever take your eyes off the ball (dog).
Scoring: The scoring of the NSUCO involves giving point values to the observations made by the clinician. The clinician will score the test for both the pursuits and saccades based on the same four factors: ability, accuracy, head movement and body movement. The manual stresses the point that the clinician should be cautioned: DO NOT ATTEMPT at first to record the performance on this by recording the number that is associated with the observed performance. Instead, record the observed performance in written form and assign numerical values later.
There are two types of observations to be scored: Qualitative (based on the clinicians qualitative judgment of performance) and quantitative (requires the clinician to count the number of times that he/she observes a particular type of behaviour). Qualitative Testing: The five qualitative aspects to be graded include:
• Head movement of pursuits
• Head movement of saccades
• Body movement of pursuits
• Body movement of saccades
• Accuracy of saccades (amount of over and undershooting) Based on a five point scale (five being the highest and one the lowest), the clinician makes an assessment as to how much of the time the patient showed motor overload type behaviours.
Quantitative Testing: There are three aspects to be counted
• Pursuit ability (the number of rotations made on pursuits)
• Saccadic ability (the number of successful round trips made on saccades
• Accuracy of pursuits (the number of target losses or refixations on pursuits) Once again, the scoring is based on a five point scale with five being the highest. The ability of pursuits is judged by the number of rotations the patient can complete without losing attention [30]. The manual cautions that “it should be emphasized that if the subject loses fixation, but then spontaneously recovers, this is not considered to be a loss of ability or attention”, but instead will be “scored as an accuracy factor”.
The saccadic ability is assessed by counting the number of successful round trips completed before total loss of attention is observed [30-33].
• Gaze limitation (n=43)
• Patients with gaze limitation: 32patients
• limitation in both vertical and horizontal gazes: 7patients limitation in vertical gaze: 18patients
• limitation in horizontal gaze: 5patients
• Binocular vision dysfunctions (n=27)Convergence insufficiency: 15 patients
• Convergence insufficiency combined with oculomotor dysfunction: 6 patients
• Convergence insufficiency combined with oculomotor dysfunction and vertical gaze palsy: 4 patients
• Intermittent divergent squint: Convergence insufficiency type: 2 patients
• Divergence insufficiency: 1 patient
• Fusional vergence dysfunction: 1 patient
Colour discrimination issues:
• Correct reading in all the Ishihara colour plates in both eyes: 24 patients
• Unable to correctly identify all Ishihara colour plates in both eyes: 2 patients
• Unable to correctly identify all Ishihara colour plates in right eye only: 2 patients
• Reading speed (measured using English text) (n=17); Mean ± SD: 86.6 ± 40 wpm; Median: 93 wpm; Range: 26 wpm-170 wpm
• Developmental eye movement test (DEM) ratio (n=6); Mean ± SD: 1.18 ± 0.40; Median: 1.22; Range: 0.46-1.67 (Figure 7,8).
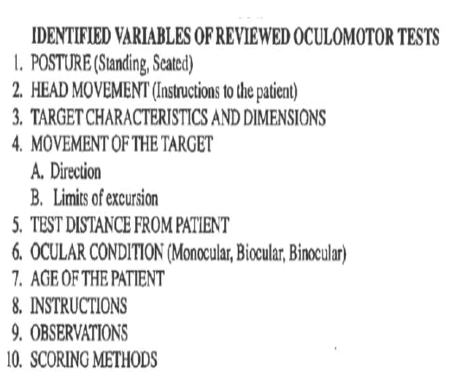
Figure 7: Identified variables of reviewed oculomotor tests.
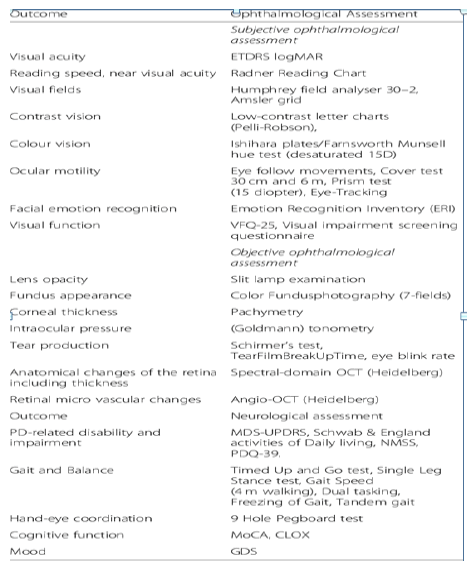
Figure 8: List of study protocol assessments.
• Gaze limitation (n=43).
• Patients with gaze limitation: 32patients.
• limitation in both vertical and horizontal gazes: 7patients limitation in vertical gaze: 18patients.
• limitation in horizontal gaze: 5patients.
• Binocular vision dysfunctions (n=27)Convergence insufficiency: 15 patients.
• Convergence insufficiency combined with oculomotor dysfunction: 6 patients.
• Convergence insufficiency combined with oculomotor dysfunction and vertical gaze palsy: 4 patients.
• Intermittent divergent squint: Convergence insufficiency type: 2 patients.
• Divergence insufficiency: 1 patient.
• Fusional vergence dysfunction: 1 patient.
• Colour discrimination issues (n=28)
)j* Correct reading in all the Ishihara colour plates in both eyes: 24 patients
)jj* Unable to correctly identify all Ishihara colour plates in both eyes: 2 patients
)jjj* Unable to correctly identify all Ishihara colour plates in right eye only: 2 patients
• Reading speed (measured using English text) (n=17); Mean ± SD: 86.6 wpm ± 40 wpm; Median: 93 wpm; Range: 26 wpm-170 wpm
•Developmental Eye Movement test (DEM) ratio (n=6); Mean ± SD: 1.18 ± 0.40; Median: 1.22; Range: 0.46-1.67
Gaze Limitation
• Patients with gaze limitation: 32 patients.
• limitation in both vertical and horizontal gazes: 7 patients limitation in vertical gaze: 18 patients.
• limitation in horizontal gaze: 5 patients.
• Binocular vision dysfunctions (n=27).
• Convergence insufficiency: 15 patients.
• Convergence insufficiency combined with oculomotor dysfunction: 6 patients.
• Convergence insufficiency combined with oculomotor dysfunction and vertical gaze palsy: 4 patients.
• Intermittent divergent squint: Convergence insufficiency type: 2 patients.
• Divergence insufficiency: 1 patient.
• Fusional vergence dysfunction: 1 patient.
• 41 Colour discrimination issues (n=28).
• Correct reading in all the Ishihara colour plates in both eyes: 24 patients.
• Unable to correctly identify all Ishihara colour plates in both eyes: 2 patients
• Unable to correctly identify all Ishihara colour plates in right eye only: 2 patients
• Reading speed (measured using English text) (n=17) Mean ± SD: 86.6 wpm ± 40 wpm; Median: 93 wpm; Range: 26-170 wpm
• Developmental Eye Movement test (DEM) ratio (n=6) Mean ± SD: 1.18 ± 0.40; Median: 1.22; Range: 0.46-1.67 (Figures 9-12)
Figure 9: Gaze limitations
Figure 10: Different convergence insufficiency.
Figure 11: Colour discrimination issues.
Figure 12: Eye movement abnormalities in parkinsonian disorders.
Other eye movement abnormalities in parkinsonian disorders
• Parkinson’s disease.
• disruption of fixation by square wave jerks in 12%
• hypsometric saccades in 9% of patients.
• impaired smooth pursuit {esp.initiation} in 4%
• increased errors in ant saccades in 6%.
• 42 oculogyric crisis in 2%.
• lid lag in 3%.
• slow vertical saccades in 43%.
• slow and hypsometric horizontal saccades in 7%.
• disruption of fixation by horizontal saccadic intrusions in 7%.
• impaired smooth pursuit both horizontal and vertical in 6%.
• preservation of slow phase of vor{fast phase lost} in 6%.
• horizontal dysconjugacy indicating(43) ino in 3%.
• loss of all eye movements{vestibular movements disappears last) in 0%.
• (44)apraxia of eyelid opening ,lid lag blepharospasm,positive (45)meyersonslight reflex)-4%.
• impaired initiation of voluntary saccade{prolonged latency, blink/head turn) supranuclear in 4%.
Symptoms
• Double vision: Dry eye due to decreased blinking OR Watery eyes was found in 49% of patients. Blurry vision in 36% of patients can also be caused by PD medication, especially anticholinergics (such as trihexyphenidyl} Trouble reading in 27 % can occur because the eye movements necessary to follow the lines of a page are slowed and have trouble starting (similar to gait freezing in the legs). Try blinking to change eye position. Levodopa can often help. Trouble voluntarily opening the eyes, known as apraxia. In 8% of cases (Figures 13-15).
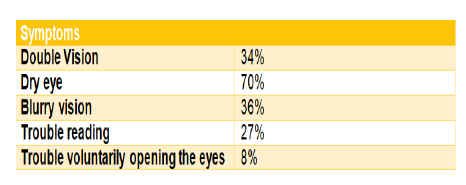
Figure 13: Symptoms
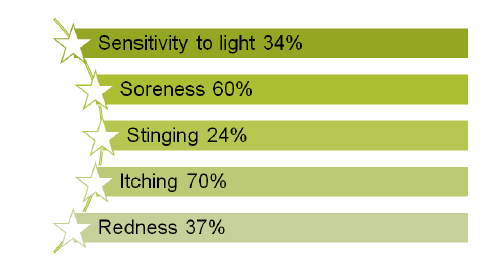
Figure 14:Treatments of botulinum toxin injections
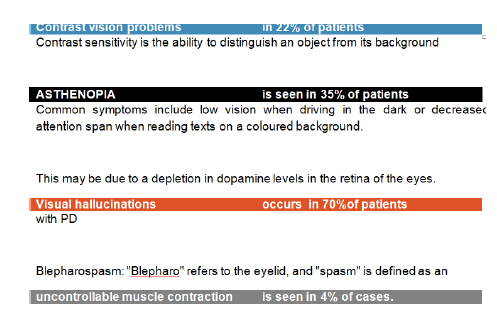
Figure 15:Results of this study.
Treatments include "lid crutches" or botulinum toxin injections sensitivity to light in 34% of cases, soreness in 60% of cases, stinging in 24% of cases, itching in 70% of cases and Redness in 37% of cases.
• Dry eyes can also result in a condition called blepharitis IN 12%. This is when the edges of the eyelid become swollen and itchy.
• Contrast vision problems, in 22% of patients, contrast sensitivity is the ability to distinguish an object from its background.
• Asthenopia-is seen in 35% of patients. Common symptoms include low vision when driving in the dark or decreased attention span when reading texts on a coloured background.
• This may be due to depletion in dopamine levels in the retina of the eyes.
• Visual hallucinations occurs in 70%of patients with PD patients reported visual hallucinations, may be caused by underreporting of visual illusions having the feeling that someone is in the room or interpreting a shadow as a person.
• Blepharospasm: "Blepharo" refers to the eyelid, and "spasm" is defined as an uncontrollable muscle contraction is seen in 4% of cases.
Other eye movement abnormalities in parkinsonian disorders:
Parkinsons disease:
• Disruption of fixation by square wave jerks in 12%.
• Hypsometric saccades in 9% of patients.
• Impaired smooth pursuit (esp. Initiation) in 4%.
• Increased errors in ant saccades in 6%.
• Oculogyric crisis in 2%.
• Lid lag in 3%.
• Slow vertical saccades in 43%.
• Slow and hypsometric horizontal saccades in 7%.
• Disruption of fixation by horizontal saccadic intrusions in 7%.
• Impaired smooth pursuit both horizontal and vertical in 6%.
• Preservation of slow phase of vor {fast phase lost} in 6%.
• Horizontal dysconjugacy indicating ino in 3%.
• Loss of all eye movements (vestibular movements disappears last) in 0%.
• Apraxia of eyelid opening, lid lag blepharospasm, positive meyersonslight reflex)-4%.
• Impaired initiation of voluntary saccadeprolonged latency, blink/head turn) supranuclear in 4%.
• Double vision in 34% of patients.
• Dry eye is seen in 70% due to decreased blinking OR Watery eyes was found in 49% of patients.
• Blurry vision in 36% of patients can also be caused by PD medication, especially anticholinergics (such as trihexyphenidyl}
• Trouble reading in 27% can occur because the eye movements necessary to follow the lines of a page are slowed and have trouble starting (similar to gait freezing in the legs). Try blinking to change eye position. Levodopa can often help.
• Trouble voluntarily opening the eyes, known as apraxia. In 8% of cases.
Treatments include "lid crutches" or botulinum toxin injections
• Sensitivity to light in 34 % of cases.
• Soreness in 60% of cases.
• Stinging in 24% of cases.
• Itching in 70% of cases.
• Redness in 37% of cases.
In PD, the most common ocular surface disorder is dry eyes (70%), which can cause decreased vision and contributes to reading difficulties. A significantly lower blink rate may lead to a less stable tear film by evaporation, causing dry eyes. This in turn may paradoxically lead to excessive blinking, watery eyes due to reflex tearing, and blepharospasm. . Regarding symptoms which had been elaborately discussed blurred vision and dry eyes are the commonest
Visual hallucinations which were observed in 70% in our study may result from the disease process itself, as well as from its treatment. Risk factors for visual hallucinations include ophthalmologic disorders, old age, drugs especially THP used in Parkinson’s disease
We found a significant negative effect of ophthalmologic symptoms on daily activities and quality of life in patients with PD. The effect of ophthalmologic symptoms may be particularly vexing for patients with PD because of their need to compensate for their motor deficits by guiding their movements visually and underlining the importance of recognizing ophthalmologic symptoms and starting early treatment when possible.
Aesthenopia is the second most common visual complaint reported in patients with PD. Due to the depletion of dopamine in the retina. The increased occurrence of reading difficulty and diplopia in our study indicates the need for a comprehensive binocular vision assessment in patients with PD.
Repka et al. reported asthenopia was present in almost 43.6% of patients aged 45 years–80 years compared to 5.1% in age-matched controls. In the study by Repka et al., 23% of patients expressed difficulty while reading compared to 26.7% in the study done by Biousse et al. which also reported reading difficulty among 9.7% in age-matched controls. Our results showed that almost 35% of PD patients encountered reading problems, higher than earlier studies.
Repka et al and Biousse et al. reported 10% (3 of 30) and 7.7% (3 of 39) of diplopia occurrence among patients with PD compared to 34% in our study, higher than previous studies. Diplopia is a well-established no motor symptom in PD. Oculomotor deficits in PD is thought to arise from deficits in oculomotor pathways in the brainstem, basal ganglia, and frontal lobes. Some may arise from dopamine depletion in the basal ganglia, causing excessive inhibition of the superior colliculus, which may lead to hypsometric eye movements. In addition, cortical involvement in the disease process of PD may affect the cortical visual areas (including the frontal and parietal eye fields), which play an important role during the initiation of eye movements, anticipation of visual stimuli, target selection, and suppression of automatic responses to visual stimuli. As with many other ophthalmologic symptoms, patients with PD generally do not report diplopia spontaneously, hence treating physicians should actively ask for double vision and other ophthalmologic symptoms.
Our data showed that 90% of patients with PD expressed at least one visual complaint that can potentially benefit from optometric management, such as reading difficulty, decreased vision, and double vision.
Factors that have been proposed to contribute to poorer acuity included decreased dopamine level, cortical dysfunction, and debilitated physical state in patients with PD. The severity of PD rather than its duration has also been found to relate to the extent of acuity reduction.
Supranuclear disorders of eye movement control have a relatively high sensitivity for detecting CNS disease, but relatively less specificity. The nuclear and infranuclear final ocular motor pathways finally control volitional movement. Other possible contributing factors include deposition of abnormally phosphorylated α-synuclein in the inner retinal layers, leading to retinal dopamine depletion and retinal dysfunction, or damage to the ganglion cell axons, leading to optic nerve head atrophy. Also, there may be a relationship between PD and neovascular macular degeneration. All these factors could cause changes in colour and contrast vision, metamorphosis, or impaired night vision. In our population, 12% of patients reported problems when reading a text on a coloured or grey background, suggesting impaired contrast sensitivity, while 8% did not see colours as brightly as before, suggesting decreased colour vision. This supports the association of impaired contrast sensitivity and decreased colour vision with the neurodegenerative process in PD. Oculomotor symptoms were also frequently reported by patients with PD.
Ophthalmologic symptoms in the intraocular and optic nerve domain were twice as frequent among patients with PD as controls. These symptoms are caused most commonly by cataract formation. This higher prevalence of symptoms possibly caused by cataract in patients with PD could result from the disease process of PD itself, since prominent α-synuclein accumulation is present in residual lens fragments of patients with PD.
Nevertheless, the high prevalence of ophthalmologic symptoms and their effect on daily life is striking, and emphasizes the need to address this subject in both research and clinical practice. Patients who report ophthalmologic symptoms need a referral for further evaluation. For those patients who do not volunteer problems themselves, a screening questionnaire such as the VIPD-Q may help with identifying ophthalmologic symptoms in PD that might otherwise be missed, thereby enabling timely referral and treatment. Further work is needed to optimize the present VIPDQ for such use in screening.
Treatment of dry eyes with eye drops is effective and has virtually no side effects. Treatments of other ocular surface disorders (e.g., blepharitis) are also non-invasive and may improve visual acuity.
Nevertheless, the high prevalence of ophthalmologic symptoms and their effect on daily life is striking, and emphasizes the need to address this subject in both research and clinical practice. Efforts to change the course of management of PD to disease modifying therapies are going on for the few decades. Among these, gene therapy is the prominent therapy. VNS is used as an adjunct therapy in PD.
[Crossref]
Citation: Kalyani, J., et al. Clinical Study On The Ocular Abnormalities In Parkinsonism. J Neuro Neurophysiol. 2023, 14 (3), 001-009
Received: 27-Feb-2023, Manuscript No. jnn-23-90249 ; Editor assigned: 27-Feb-2023, Pre QC No. jnn-23-90249 (PQ); Reviewed: 02-Mar-2023, QC No. jnn-23-90249 (Q); Revised: 03-Mar-2023, Manuscript No. jnn-23-90249 (R); Published: 30-Mar-2023, DOI: 10.35248/2332-2594.23.14(3).342
Copyright: © 2023 Kalyani, J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.