Case Report - (2020) Volume 6, Issue 4
We report the case of a 70-year-old frail patient with AML-M0, normal karyotype and negative FLT3 and NPM1, treated successfully with the combination of Azacitidine (75 mg/m2 D1-D7) and Venetoclax (100 mg D1-D14) in whom Fluconazole was added to potentiate the effect of Venetoclax in vivo, reaching a Complete Remission. Venetoclax is metabolized by the CYP3A. When administered with strong CYP3A inhibitors, such as azoles, drug’s concentration will increase by 2-7 folds empowering its effect. Considering the inadequate accessible dose of Venetoclax and the current Lebanese economic crisis, 200 mg of daily Fluconazole, a moderate CYP3A4 inhibitor, was combined to Venetoclax to increase its bioavailability. The patient reached a complete remission after four cycles. He presented grade I febrile neutropenia after the first two cycles. No major adverse events were reported. We believe it’s the first case reporting a complete remission after combining low dose Venetoclax, Azacitidine and a moderate CYP3A inhibitor.
Acute myeloid leukemia • Venetoclax • Azacitidine • Fluconazole • Complete Remission
Acute Myeloid Leukemia (AML) is the most common acute leukemia in adults with an increasing incidence with age [1]. While treatment development improved outcomes in younger patients, results in the elderly population are still dismal [2]. Patients older than 65-years, usually carrying multiple comorbidities, present more with secondary-AML and unfavorable cytogenetic, thus, respond less to standard intensive chemotherapy and develop more treatment related side effects [1]. Consequently, treatment approach is usually based on less intensive strategies such as Hypo-methylating Agents (HMA), Azacitidine or Decitabine, and Low dose Cytarabine (LDAC) which provides brief Overall Survival (OS). Recently, it has been demonstrated that BCL-2 (B cell lymphoma-2), a regulatory protein that mediates tumor cell survival, is over-expressed in patients with AML and confers chemo-resistance. Therefore, the addition of BCL- 2 inhibitor to standard treatment may reinitiate the apoptosis and lead to better outcomes [3]. Venetoclax is metabolized by the CYP3A, meaning that strong CYP3A inhibitors, such as anti-fungal, may lead to an increase in the drug’s concentration by 2 to 7 folds. This is the case of azoles, such as fluconazole, when added to Venetoclax may empower its effect [4].
However, this scientific optimism is rapidly tempered by worries concerning access, affordability and financial burden [5]. In Lebanon, most oral innovative drugs are rapidly available in the market after their approvals but patient access to drugs may not be as easy. Different financing and funding systems exist according to the patient coverage. So, when it comes to oral therapies, the patient can bear a heavy portion or even all the cost of the drug by his own leading to an unanticipated financial distress. Moreover, Lebanon is suffering, currently, an unprecedented economic crisis making the financial burden heavier on the patient.
For that, we will report, in what follows, the case of a 70-year-old frail patient with AML treated successfully with the combination of Azacitidine with modified dose of Venetoclax due to inaccessibility and refunding issues, in whom Fluconazole was added to potentiate the effect of Venetoclax in vivo, who reached a Complete Remission after four cycles of treatment.
Mr. MB, a 70-year-old male patient previously hypertensive with a history of autoimmune thyroiditis treated with steroids, admitted to the emergency room complaining of general weakness starting few weeks ago. The blood test showed pancytopenia with hemoglobin level of 7.4 g/dL, WBC 1800/mm-3 and platelet count of 32 × 109/L. Peripheral blood smear examination showed around 20% of blasts suggestive of acute leukemia. An urgent bone marrow aspirate was done showing 30% of blasts confirming the diagnosis of Acute Myeloid Leukemia type M0 (AML-M0) (Figure 1). The leukemic cells flow cytometry was positive for CD34, CD117, HLADR and negative for CD13, CD33, CD19, CD3, CD15, CD14, CD64. The patient’s karyotype was normal with negative FLT3 and negative NPM1.
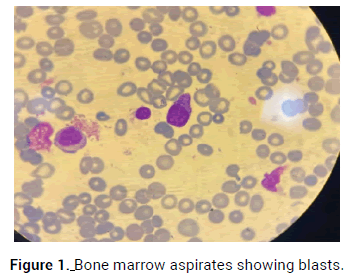
Figure 1. Bone marrow aspirates showing blasts.
According to the American Society of Clinical Oncology (ASCO) guidelines, the patient’s Geriatric Assessment (GA) was done using the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) [6]. He had a total of 10 points putting him in the high score risk category precluding intensive induction chemotherapy. Therefore, the current standard of care treatment for elderly unfit patients was proposed for the patient, combining Azacitidine (75 mg/m2 from D1 to D7 every 28-days) with Venetoclax (initial ramp up phase followed by 400 mg PO daily).
In fact, the third part payer of the patient delivered Venetoclax at a prefixed lower dose of 100 mg PO per day from day 1 to day 14, noncompliant with the current approved dosage. It was combined with Azacitidine 75 mg/m2 per day from day 1 to day 7 and Fluconazole at a dose of 200 mg orally per day from day 1 to day 14. One cycle repeated every 28 days.
The patient was admitted to the hospital for the administration of Azacitidine for 7 days every cycle and for monitoring. Although he was at low risk of developing Tumor Lysis Syndrome (TSL), adequate hydration and uric acid lowering agents was administered. Progressively, the patient started showing clinical and hematological improvement with a clear decrease in transfusion needs.
A new bone marrow aspirate was done after 4 cycles showed a complete remission with slight dyserythropoiesis as well as complete hematologic remission. The Minimal Residual Disease (MRD) was not done since it was not available at our center (Figure 2).
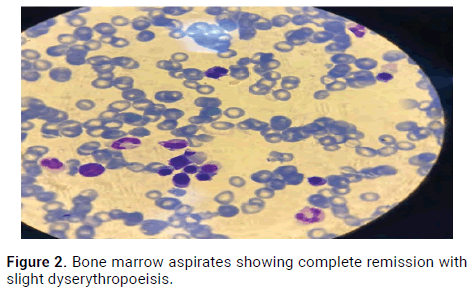
Figure 2. Bone marrow aspirates showing complete remission with slight dyserythropoeisis.
Until the submission of the article the patient received a total of 6 cycles and still in complete remission.
Low-dose Cytarabine (LDAC) and Hypomethylating Agents (HMA) such as Azacitidine or Decitabine have been the standard of care for elderly AML patient’s ineligible for intensive induction chemotherapy regimens. With low median survival rates, outcomes were still dismal [7].
Venetoclax, an oral and potent B-cell lymphoma 2 (BCL-2) inhibitor, when added to LDAC or HMA in frontline setting, demonstrated in a phase Ib study, published in 2019, an encouraging Overall Response Rate (ORR) of 68% and a leukemia response rate of 83% [8]. This was confirmed by the results of the phase III VIALE-A study in 2020 that showed a statistically significant improvement in overall survival and duration of remission when Venetoclax was added to Azacitidine in treatment naive AML patients, ineligible for intensive therapy [9,10].
Venetoclax is metabolized mainly by the CYP3A4 enzyme and, to a lesser degree, by the p-glycoprotein. Therefore, its concomitant use with food or drugs that inhibit the CYP3A4 will increase its plasma concentration by 2 to 7 folds [10]. Fluconazole, a triazole antifungal drug commonly used in AML patients, is considered a moderate CYP3A4 inhibitor. When combined to Venetoclax, it increases the latter maximum concentration in the plasma as well as the Area under the Curve (AUC) by two to five folds prolonging the patient’s drug exposure [11]. That’s why it is recommended to reduce the dose of Venetoclax by around 50% when used in concomitance with Fluconazole to maintain safe therapeutic ranges preventing unwanted adverse events [7]. Moreover, Azacitidine metabolism is not mediated by the CYP enzymes; therefore, no effects were noticed when administered along with Fluconazole [12].
Based on Venetoclax pharmacokinetics and considering the inadequate accessible dose of Venetoclax by the patient’s third party (100 mg daily instead of 400 mg) along with the current Lebanese economic crisis that preclude additional cost on the patient, we decided to combine 200 mg of daily Fluconazole to the prefixed low dose Venetoclax in order to increase its bioavailability in the patient’s plasma and to increase his chance of response. This schema was extrapolated from the results of the phase Ib sub-study evaluating Venetoclax with posaconazole interaction in untreated AML patients where the results showed an Overall Response Rate (ORR) of 67% [13]. Furthermore, the drug’s safety was comparable with no increased side effects related to the addition of CYP3A inhibitor as it was shown in the phase Ib/II study where 50% of the patients treated with Venetoclax combined to LDAC were receiving posaconazole [14]. In fact, the patient reached a complete remission upon the end of the fourth cycle. He presented grade I febrile neutropenia after the first two cycles treated adequately according to recommended protocols. No major adverse events were reported.
To date, we believe it’s the first case described in the literature, reporting a complete remission after combining low dose Venetoclax and Azacitidine to a moderate CYP3A inhibitor.
Venetoclax combined to HMA or LDAC increased the likelihood of achieving a complete remission in elderly AML patient ineligible for intensive therapies. However, access to costly oral drugs can financially exhaust the patient especially in countries facing economic crisis such as Lebanon. Our experience showed that adding a CYP3A4 inhibitor to Venetoclax at lower doses was as effective as treating the patient with the recommended ones. Thus, while decreasing the financial burden of the treatment, we tried to ensure, as much as possible, the same outcome and drug safety.
In the near future, with the rapid expansion of new innovative cancer drugs and their incorporation in the treatment guidelines, we will be concerned, as clinicians, on the burden of their cost and accessibility with their consequent financial toxicity on the patient. For that, we believe our case may open the floor for future prospective randomized clinical trials investigating the efficacy and tolerability of such combination.
Citation: Zeghondy J, et al. Azacitidine Combined with Low Dose Venetoclax and Azole in Treatment-Naive, Elderly Patient with Acute Myeloid Leukemia: A Case Report. Oncol Cancer Case Rep, 2020, 06 (4), 001-003.
Received: 05-Sep-2020 Published: 06-Oct-2020, DOI: 10.35248/2471-8556.20.6.001-003
Copyright: © 2020 Zeghondy J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.