Journal of Multiple Sclerosis
ISSN - 2376-0389NLM - 101654564
Review Article - (2021) Volume 8, Issue 10
Fatigue in Multiple Sclerosis [MS] is one of the most disabling symptoms having a negative impact on disability scores and on Health-Related Quality of Life [HRQOL] occurring more frequently and with increasing severity in patients with primary or secondary-progressive disease compared to those with a relapsing-remitting presentation. With the advent of modern neuroimaging and spectroscopic methods, new insights into the pathophysiology of fatigue in MS are starting to appear in support of predominantly centrally-mediated changes. For example, abnormalities of motor-evoked potentials [MEPs] and the burden of Magnetic Resonance Imaging [MRI] lesions are independently correlated with fatigue severity in patients with MS consistent with a central origin. Moreover, the subsequent use of 18-Fluorodeoxyglucose Positron Emission Tomography [PET] and 1-H-Magnetic Resonance Spectroscopy [MRS] were employed to demonstrate that metabolic dysfunction in specific brain regions such as frontal cortex and basal ganglia were implicated and that a striatalthalamic- frontal cortical network may play a key role in MS-related fatigue. Major hypotheses involving an imbalance in the dopamine systems in the CNS or of the pro-inflammatory cytokine TNFa were proposed as key central mediators. Electronic and manual searches of databases including PubMed, Medline, Embase and relevant journals using appropriate keywords yielded 6 systematic reviews on amantadine for fatigue in MS, 2 of which had associated meta-analyses. The reviews were based on the results of 11 Randomized Controlled Trials [RCTs], 8 of which were placebo-controlled. Six trials compared the efficacy of amantadine with other active agents. Beneficial effects of amantadine were reported in 9/11 trials compared to placebo for MS patients with fatigue of either chronic progressive or relapsing remitting phenotypes. Amantadine was rated superior to modafinil [2 trials], pemoline [1 trial], Acetyl-L-Carnitine [ALCAR, 1 trial], and ondansetron [1 trial] for fatigue treatment. Doses of amantadine shown to be effective for the treatment of fatigue in MS were generally in the 100-200 mg/d for periods of 4 weeks. Clinical Practice Guidelines published by NICE [UK] in 2019 and The German MS Society concluded that amantadine was recommended for the pharmacological management of MS-related fatigue. An important highlight of the present review came in the form of the results of a key study based upon a novel standardized repetitive fatiguing task in patients with MS which resulted in the identification of the precise mechanism by which amantadine exerts its beneficial effects on fatigue in these patients. Treatment with amantadine [200 mg/d, 3 months] led to significant improvements in levels of intracortical inhibition of motor cortex by a process involving the re-balancing of GABA: Glutamate ratios and consequent improvements of central fatigue scores.
Amantadine, Multiple sclerosis, Fatigue, NMDA receptor antagonists, Neuroimaging, Positron Emission Tomography, MRI
Fatigue is commonly encountered in MS occurring in over 75% of patients in whom, for many, it represents the most disabling symptom having a negative influence on disability scores and on quality of life. Fatigue occurs more frequently and with increasing severity in patients with primary or secondary-progressive disease compared to those with a relapsing-remitting presentation [1].
Fatigue in MS is an overwhelming feeling of tiredness and lack of energy progressing to exhaustion. It may occur independently of mood disturbances but is aggravated by stress. It can occur at any stage of MS but is often more severe and more frequent in patients with primary or secondary progressive disease compared to relapsing-remitting disease [2]. Although peripheral mechanisms have been implicated in the pathogenesis of fatigue in MS, there are clear indications that central abnormalities are additionally [or even preferentially] responsibleFatigue in MS is an overwhelming feeling of tiredness and lack of energy progressing to exhaustion. It may occur independently of mood disturbances but is aggravated by stress. It can occur at any stage of MS but is often more severe and more frequent in patients with primary or secondary progressive disease compared to relapsing-remitting disease [2]. Although peripheral mechanisms have been implicated in the pathogenesis of fatigue in MS, there are clear indications that central abnormalities are additionally [or even preferentially] responsible.
A number of grading scales, both uni- and multi-dimensional, have been developed for the assessment of fatigue severity in a range of disorders including MS (Table 1). Of these, the Fatigue Severity Scale [FSS] is one of the most commonly used for measuring fatigue’s impact on daily living rendering it commonly used in clinical trials [3]. The Fatigue Impact Scale [FIS] on the other hand assembles 40 items including cognitive, psychosocial and physical dimensions; it is the most widely employed multidimensional scale for assessment of fatigue severity in MS.
| Scale | Dimensions | Number of items |
|---|---|---|
| Multidimensional | ||
| Fatigue Imact Scale [FIS] | Cognitive, psychosocial, physical | 21 |
| Multidimensional assessment of fatigue [MAF] | Severity, timing, distress, interference | 16 |
| Multidimensional fatigue inventory [MFI] | General activity, physical, motivation | 24 |
| Fatigue Scale [FS] | Physical, mental | 14 |
| Unidimensional | ||
| Fatigue Severity Scale [FSS] | Severity on daily living | 9 |
| Functional assessment of MS [FAMS] | Tiredness-thinking subscale | 9 |
| Rand Index of Vitality [RIV] | Vitality | 4 |
Table 1: Commonly-employed scales for assessment of MSRF [2,3].
Central versus peripheral mechanisms
Peripheral mechanisms are implicated to some extent in the pathogenesis of fatigue in MS as demonstrated by Magnetic Resonance Spectroscopy showing that, for example, phosphocreatine re-synthesis following exercise is slowed in patients with MS resulting from disuse and/or deconditioning [4]. However, the advent of new technologies continues to support the notion that the predominant role is played by centrallymediated changes.
For example, Motor-Evoked Potentials [MEPs] and Magnetic Resonance Imaging [MRI] were used to investigate the pathophysiology of fatigue in patients with MS with [n=15] or without [n=15] fatigue the severity of which was scored by FSS. Patients were matched for age, gender, disease duration and Expanded Disability Status Scale scores. MEPs were found to be abnormal in five patients with fatigue and in one patient without fatigue and a significant association was observed between FSS scores and the burden of MRI lesions [r=0.5, p<0.005]. Findings were thus consistent with a CNS origin of fatigue in MS patients [5].
Role of specific brain regions and their functional connectivity
18F-Fluorodeoxyglucose Positron Emission Tomography [PET] was used to estimate cerebral glucose metabolism [CMR-glucose] in 47 MS patients and, making use of the FSS for assessment of fatigue severity, a comparison was made between fatigued [FSS>4.9, n=19] and nonfatigued [FSS<3.7, n=16] groups. Healthy subjects [n=16] served as controls. Global CMR-glucose was significantly decreased in MS but there was no correlation with fatigue severity. On the other hand, fatigue severity was inversely related to bilateral decreases of CMR-glucose in the prefrontal area involving the lateral and medial prefrontal cortices as well as in the premotor cortex, putamen and lateral head of the caudate nucleus leading the authors to conclude that fatigue in MS is primarily associated with frontal cortical and basal ganglia dysfunction [6].
Proton Magnetic Resonance Spectroscopy [1H-MRS] has also been used to measure metabolites and metabolite ratios [NAA/Cr, NAA/Cho, CHO/ Cr] in basal ganglia of 41 patients with relapsing-remitting MS and mild disability compared to 20 healthy controls. Fatigue was assessed by FSS in all cases. Patients were classified as fatigued with FSS of 5.0 or more or non-fatigued with FSS of <4.0. Significant decreases of NAA/Cr were observed in patients with fatigue consistent with the notion that basal ganglia metabolic dysfunction contributes to MSRF [7].
A subsequent report observed that cognitive fatigue in MS was associated with increased activation in the basal ganglia brain and frontal lobes using the technique of functional MRI in patients with moderate-tosevere MS [8]. Members of the same team later examined “state and trait” aspects of cognitive fatigue using combined fMRI and DTI. The findings illustrated the importance of the role of a striatal-thalamic-frontal cortical network for the understanding of mechanisms implicated in MS-related fatigue [9]. Interestingly, elements of this network have been proposed to underpin mechanisms responsible for fatigue in other clinical populations including Parkinson’s disease [10] and stroke [11]. Moreover, in line with the focus of the present review, dysfunction and frank pathology have been repeatedly described in striatum and thalamus as well as frontal and parietal cortices in association with self-reported fatigue in patients with MS [6,7,12].
In a study designed to assess the role of white matter [WM] and grey matter [GM] lesions and global versus regional damage and atrophy in relation to fatigue in MS, dual-echo, double inversion-recovery, highresolution T1-weighted and diffusion-tensor MRI was used in fatigued MS patients (n=31), non-fatigued MS patients (n=32) and control subjects [n=35]. It was demonstrated that, although global brain damage did not differ between fatigued and non-fatigued patients, damage to strategic WM and GM regions characterized by microstructural abnormalities and atrophy would likely have contributed to the pathogenesis of fatigue in MS [13].
Using resting state f-MRI, diffusion tensor imaging and voxel-based morphometry in relapsing-remitting MS in 44 patients vs 20 age-matched controls revealed alterations of both basal ganglia volumes and functional connectivity but with no correlation with volume or fatigue severity. Interestingly there was a significant negative correlation between fatigue severity and functional connectivity of basal ganglia nuclei but changes in medial prefrontal cortex, caudate nucleus and motor cortex were independent of overall disability. Moreover, the pattern of the connectivity changes was suggestive of disruption of basal ganglia function [14].
Other mechanisms have been invoked that reinforce the notion of a cortical origin of fatigue in MS that include dysfunction of inhibitory intracortical mechanisms, increased corticomotor excitability and decreased recruitment of corticospinal neurons [15].
The dopamine imbalance hypothesis
Neuroimaging and spectroscopic studies summarized above reveal a high degree of brain regional selectivity in terms of cellular damage, atrophy and energy metabolism. Moreover, these structural and functional impairments occur in regions of the brain that are heavily innervated by dopaminergic neurons such as the basal ganglia [striatum, pallidum, caudate] and frontal/prefrontal cortex. Consequently, it is not surprising that a great deal of attention has been focussed on the dopamine [DA] system and its interconnectivity with other CNS networks.
DA is synthesized in the brain in one of two subcortical regions namely the sustantia nigra [SN] and the ventral tegmental area [VTA] from which originate the nigrostriatal pathway linking the SN with the striatum and the mesocorticolimbic pathway projecting from the VTA to the striatum and prefrontal cortex. Functional neuroimaging studies highlight the involvement of the mesolimbic DA pathway in patients with fatigue related to brain injury and, with relevance to MS in particular, one study showed that such patients with higher than average FSS scores manifested significant decreases of cerebral energy metabolism in striatum and prefrontal cortex compared to non-fatigued individuals [6]. More recently, reports of reduced mesocorticolimbic connectivity in relation to fatigue in patients with MS have appeared [14,16]. Together, these findings have resulted in the formulation of what has become referred to as “The dopamine imbalance hypothesis of fatigue in multiple sclerosis” [17].
The immune system
Proinflammatory cytokines such as tumour necrosis factor alpha [TNFα], interferon gamma [IFNγ] and the interleukins IL-6 and IL-10 are reportedly increased in a range of disorders linked to fatigue including viral infections, some cancers and in chronic fatigue syndrome. A study of 37 patients with relapsing-remitting [n=29] or secondary progressive [n=8] MS aged 41.0 +/- 10.2 yrs. measured cytokine mRNA for IFNγ, TNFα or IL-10 by RT-PCR with severity of fatigue assessed by FSS. Significant increases of serum TNFα mRNA in patients with fatigue [FSS>4.0, n=26] or FSS >5.0, n=14 were observed compared to MS patients without fatigue [FSS<4.0, n=11]. No differences were noted for IFNγ or IL-10. It was concluded that the findings were consistent with a selective role for TNFα in the pathogenesis of fatigue in both relapsing-remitting and secondary progressive MS [18].
Efficacy of Amantadine for the Treatment of Fatigue in Multiple Sclerosis: The Evidence Base
Systematic reviews/ meta-analyses [listed by order of date of publication of results]
Branas et al. 2000 In this study, only two drugs, amantadine and pemoline met inclusion criteria for full systematic review. In the case of amantadine, one parallel and three crossover trials involving 236 patients with MS were identified. All studies showed a pattern in favour of amantadine compared to placebo, but studies were open to bias and, although only amantadine appeared to have proven ability to alleviate symptoms of fatigue in MS, it was concluded on the basis of the quality of the evidence that only a limited number of MS patients would likely derive long-term benefit. It was concluded that additional in-depth studies were now required [19].
Pucci et al. 2007 The review was based on the results of Randomized Controlled Trials [RCTs] of the efficacy of amantadine for fatigue in MS identified using electronic searches of MEDLINE, EMBASE, manual searches of relevant journals, reference lists in order to determine the effectiveness and safety of amantadine for reducing fatigue in MS patients [20]. Inclusion criteria were RCTs vs placebo or another agent. The review appears to have been an updated version of an earlier review by members of the same group [21]. Meta-analysis was not performed due to inadequate data and heterogeneity of outcome measures.
Four trials [1 parallel, 3 crossovers] were identified. The overall quality of trials was poor with a high probability of bias for a total of 236 MS patients with fatigue. Amantadine treatment was well tolerated resulting in small but somewhat inconsistent improvements in fatigue. Side effects were generally mild in 10-50% of patients in both amantadine and placebo treatment groups
Asano and Finlayson 2014 RCTs describing the use of amantadine were searched electronically via PubMed and Embase together with manual searches of original articles revealed 5 trials of amantadine [3 vs placebo; 1 vs aspirin, 1 vs ALCAR] in which, in all cases, risk of bias was assessed as low [22]. Data from Forest Plots of studies by Ashtari, Krupp and by The Canadian MS Research Group (CMSRG) provided evidence of significant differences favouring amantadine over placebo (Figure 1) with ES and 95% CI values as follows:
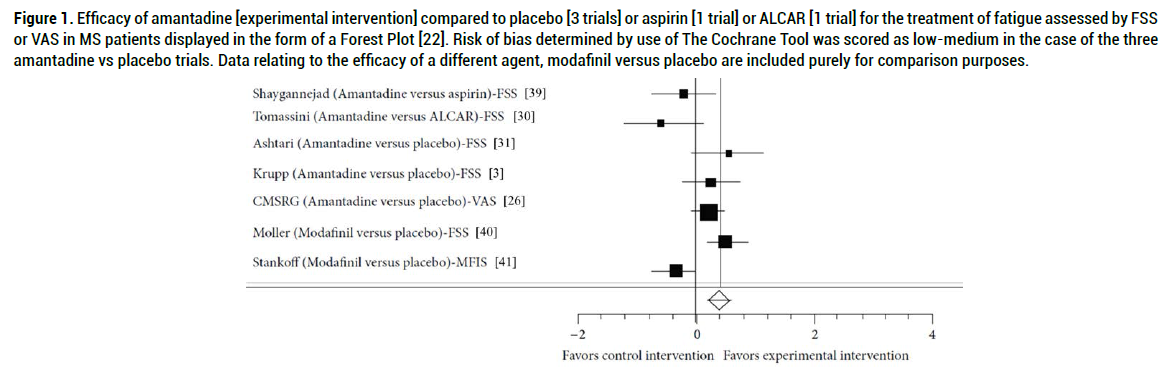
Figure 1. Efficacy of amantadine [experimental intervention] compared to placebo [3 trials] or aspirin [1 trial] or ALCAR [1 trial] for the treatment of fatigue assessed by FSS or VAS in MS patients displayed in the form of a Forest Plot [22]. Risk of bias determined by use of The Cochrane Tool was scored as low-medium in the case of the three amantadine vs placebo trials. Data relating to the efficacy of a different agent, modafinil versus placebo are included purely for comparison purposes.
Ashtari ES: 0.55 95% CI: -0.06 to -1.16
Krupp ES: 0.24 95% CI: -0.23 to -0.73
CMSRG ES: 0.21 95% CI: -0.08 to -0.51.
Yang et al. 2017 Data from 11 RCTs for 723 patients were identified based on searches of PubMed, Embase, Medline, Google Scholar and Cochrane Library in which comparisons were made between amantadine, modafinil, aspirin, ALCAR, pemoline, 4-aminopyridine for the treatment of fatigue in MS. All drugs were found to be safe except pemoline. Meta-analysis showed benefit for amantadine but not for modafinil. Benefit for ALCAR, aspirin, 4-aminopyridine was not analysed due to low patient numbers. It was concluded that amantadine was the only effective agent with sufficient evidence for the treatment of fatigue in MS [23].
Miller and Soundy 2017 Five systematic reviews were identified related to pharmacological interventions [Amantadine, Prokarin, Modafinil, Permoline, Carnitine] aimed at the treatment of fatigue in patients with MS. The overall methodological quality of the reviews was reflected in an AMSTAR score of 6.5 [SD: 1.87: 95% CI: 5.75-7.25]. For amantadine, the results of 5 published reviews were included three of which reported some degree of fatigue improvements based upon the results of 5 trials although some results were conflicting. There was evidence of potential benefit of Modafinil but no evidence of benefit in the case of Pemoline or Carnitine. Significant side effects of amantadine were reported in 2/5 trials [24].
Perez et al. 2020 Data from 11 RCTs and 531 participants included based on searches of PubMed, Embase, Scopus, Cochrane Libr, Science direct, ClinicalTrials.gov. Agents included amantadine, ALCAR, aspirin, ondansetron, placebo. Variety of validated scales for MSRF. Results: amantadine the best-studied agent for MSRF with benefit of amantadine for MSRF at doses of 100mg bid or 200mg/d. Most common side effects: insomnia, nausea, constipation, anxiety. Limited evidence was provided for the effect of co-administration with other agents or for long-term use of amantadine [25].
Randomized controlled trials (Listed by order of date of publication of results)
CMSRG 1987 115 MS patients with chronic persistent fatigue were recruited in this10 week placebo-controlled crossover trial with a 2-week baseline followed by 2x 3-week treatment periods separated by a 2-week washout. Treatment involved amantadine [100mg bid] compared to matching placebo. Fatigue scores were measured daily making use of a 50mm Visual Analogue Scale. Randomization was computer generated. Fatigue scores, both patient and physician ratings and physical activities of daily living were all improved by amantadine. consistent with beneficial effects on fatigue in MS [26].
Plaut 1987 34 MS patients completed this placebo-controlled trial on the effect of amantadine [200mg/d for 1 month] Significantly lower mean MFIS scores were observed compared to placebo with MD: 17.3, p=0.001. Amantadine was found to be superior to modafinil or ALCAR. Neurological status and disability scores were unchanged by amantadine, but rates of relapse appeared to be reduced [27].
Rosenberg and Appenzeller 1988 [28] 10 MS patients completed this double-blind, placebo-controlled, crossover trial. Amantadine HCl was effective in 6/10 [Mean Kurtzke scale scores: mean:3.3, range: 0-7 accompanied by significantly increased levels of beta-endorphin.
Cohen and Fisher 1989 [29] A randomized double-blind, crossover study was conducted to evaluate the efficacy of amantadine hydrochloride in treating 29 patients with MS-associated fatigue. According to patients' daily diary ratings, amantadine [100mg bid for 4 weeks followed by 2 week washout period prior to crossover] produced small but statistically significant improvements in fatigue across four of seven dimensions (overall energy level, concentration, problem solving, and sense of well-being). In addition, patients with MS who were taking amantadine performed slightly better on the Stroop Interference Test, an attentional measure of freedom from distracting information. Although retrospective reports by patients with MS did not confirm the degree of improvement recorded on a daily basis, the study's results suggested that amantadine may offer modest benefits in alleviating the day-to-day subjective experience of fatigue and, when the seven measures of fatigue were collapsed to produce one overall fatigue score, there was a trend toward improved overall functioning with the amantadine (F =4.01 [1.21], p=0.058.
Krupp et al. 1995 Amantadine, pemoline and placebo were compared in this double-blind, randomized [3], parallel trial in 93 ambulatory MS patient with fatigue assessed by Fatigue Severity Scale [FSS] and MSspecific fatigue scale [MS-FS] Results: Amantadine resulted in significant reductions in fatigue by MS-FS versus placebo [p=0.04] (Figure 2). Verbal reporting at the end of the study revealed that 79% of patients preferred treatment with amantadine vs 52% treated with placebo with p= 0.03. Benefits of amantadine were not due to changes in sleep, depression or neurologic disability.
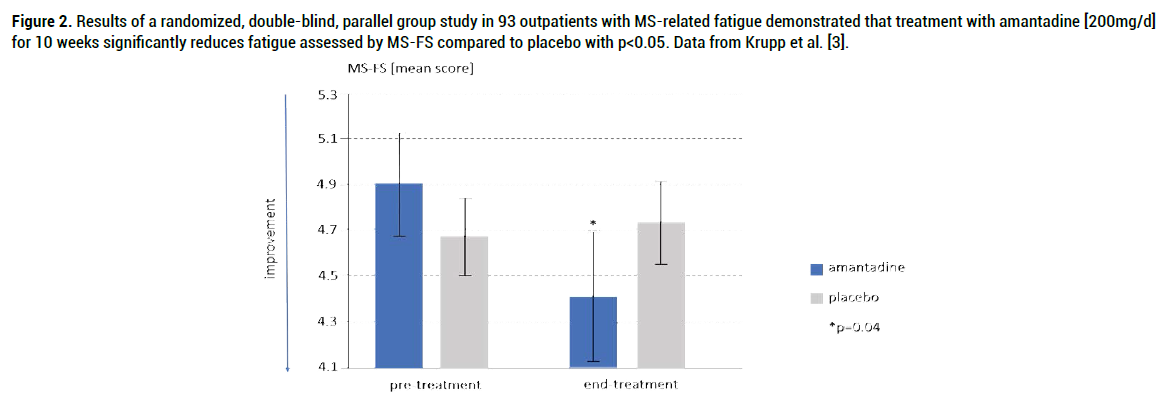
Figure 2. Results of a randomized, double-blind, parallel group study in 93 outpatients with MS-related fatigue demonstrated that treatment with amantadine [200mg/d] for 10 weeks significantly reduces fatigue assessed by MS-FS compared to placebo with p<0.05. Data from Krupp et al. [3].
Tomassini et al. 200 This randomized trial included 36 MS patients with fatigability assessed by FSS questionnaire assigned to amantadine [100mg bid] vs ALCAR [1g bid] in a crossover design [3-month treatment (1) then 3-month washout then 3-month treatment (2), there was no placebo group]. Results: ALCAR was found to be more effective than amantadine by FSS with p=0.039. Amantadine was less well tolerated; side effects: nausea, dizziness [30].
Ashtari et al. 2009 This randomized placebo-controlled prospective study included 42 MS patients with fatigue assessed by FSS at baseline and 2 months after treatment with amantadine [200mg/d] or placebo. A significant decrease in FSS was observed following amantadine compared to placebo with p<0.05 [31].
Ledinek et al. 2013 Efficacy of amantadine [200mg/d, 1 month] vs placebo or two other agents [modafinil (200 mg/d), ALCAR(2 g/d)] in 60 MS patients with fatigue [39 female] aged 38 ± 6.7yr with disability level of <5.5 on the Kurtzke Expanded Disability Status Scale [EDSS]. Efficacy of treatment was assessed by Modified Fatigue Impact Scale [MFIS] Mean duration of MS was 6.6 ± 1.2yr. A 1-month treatment with amantadine improved fatigue in patients with relapsing-remitting MS as evaluated by MFIS compared to placebo [MD: 12.4, p=0.05 with Keppel-corrected alpha of 0.046] together with improvements in HRQOL. No benefit or nonsignificant trends were observed for modafinil or ALCAR [32].
Santarnecchi et al. 2015 This is the first study to directly address an important issue namely the neurophysiological effects of amantadine that underpin its beneficial effects on central fatigue in MS patients. In a randomized crossover trial, 10 female patients with either relapsingremitting (n=8) or progressive (n=2) MS and expanded disability status scale scores from 1.0 to 5.5 were enrolled in the trial [15]. The control group consisted of 10 healthy volunteers [3 male, 7 females]. Investigators measured cortical functional changes induced by a standard fatiguing repetitive tapping task in which the Cortical Silent Period [CSP] was recorded by two different hand muscles. Chronic treatment with amantadine (200mg/d for 3 months) led to improvements in fatigue scale scores such as FSS with p<0.012 and CSP duration recorded in MS patients before and during chronic treatment with amantadine were significantly correlated with improvements in FIS scale scores [p=0.039, r=0.673]. It was suggested that amantadine via its NMDA antagonist properties induced an increase in the physiological patterns of neural pools responsible for CSP resulting in a re-balancing of the dysfunctional GABA/glutamate balance at the cortical level due to central fatigue. In summary, the investigators demonstrated that chronic treatment with amantadine restored physiological levels of intracortical inhibition in the motor cortex that was associated with an improvement of central fatigue scores.
Khazaei M et al. 2019 In a randomized crossover trial, 53 MS patients [45 female] were randomly assigned to one of two groups, ondansetron [4mg bid] or amantadine [100mg bid] for 4 weeks each followed by a 2-week washout then crossover for 4 further weeks. Assessment of fatigue severity made use of the FSS questionnaire. Each drug significantly decreased fatigue severity [p<0.001] with no significant difference between the two. However, when ranking the severity of fatigue [mild/ moderate/severe] improvements using amantadine were judged to be better than those of ondansetron with p=0.026 [33].
Nourbakhsh et al. 2021 In a randomized placebo-controlled trial, 141 MS patients with fatigue and MFIS of >33, the efficacy of amantadine [up to 100mg bid] vs placebo or one of two other agents [modafinil [up to 100mg bid] or methylphenidate [up to 10mg bid] were compared [34]. Patients were randomly assigned to one of four medication administration sequences namely amantadine/placebo/modafinil/methylphenidate or the three other possible sequences. All patients received all four medications with a 2-week washout between each. Fatigue severity was estimated by Modified Fatigue Impact Scale [MFIS]. Results: MFIS @ baseline: 51.3 [49.0-53.6], placebo: 40.6 [38.2-43.1], amantadine: 41.3 [38.8-43.7]. Using this somewhat complex design, none of the agents [amantadine, modafinil or methylphenidate] were superior to placebo in improving MS-related fatigue as assessed by MFIS. AEs with amantadine: 39% vs 31% in placebo with 2 serious AEs reported with amantadine [pulmonary embolism, myocarditis].
Clinical practice guidelines
According to the report published in 2017 [24], amantadine was the only drug recommended by NICE [UK] for the pharmacological management MS-related Fatigue. It is important to point out that NICE Guidelines also advise a wide range of non-pharmacological procedures including education [energy conservation], exercise [aerobic, resistance and yoga], psycho-behavioural techniques [Cognitive Behavioural Therapy (CBT) or mindfulness programs such as tai-chi, yoga, relaxation and meditation] all of which have the potential to lead to significant improvements in MSRF.
Websites from different health authorities or MS societies with Clinical Practice Guidelines or recommendations for treatment with respect to fatigue in MS include the following:
1. MS society, UK: https://www.mssociety.org.uk/about-ms/ signs-and-symptoms/fatigue/managing-fatigue (see section: 9. What drug treatments might help with MS fatigue?)
NICE guideline CG186, 2014, UK: [National Collaborating Centre for Chronic Conditions (UK). Multiple Sclerosis: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2004. Updated version: NICE Clinical Guidelines, No. 186; London: National Institute for Health and Care Excellence (UK); 2019 Nov.] https://www.ncbi. nlm.nih.gov/books/NBK552607/?report=reader. Fatigue 1.5.4. Offer amantadine to treat fatigue in people with MS (At the time of publication (October 2014), amantadine did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented.) https://www.nice.org.uk/guidance/ cg186/resources/multiple-sclerosis-management-of-multiple-sclerosisin- primary-and-secondary-care-35109816059077.
According to The German Society of Multiple Sclerosis treatment with amantadine in daily doses of 2x100mg may be at least partially successful whereas an alternative agent modafinil is not approved for the treatment of MS-related fatigue in Germany. See: https://www.dmsg.de/multiplesklerose- infos/ms-behandeln/symptomatische-therapie/fatigue/
National multiple sclerosis society, US: Amantadines sometimes effective in relieving fatigue in multiple sclerosis. The usual dosage for the management of fatigue in MS is 100 to 200 mg daily, taken in the earlier part of the day in order to avoid sleep disturbance. https://www. nationalmssociety.org/Treating-MS/Medications/Amantadine
Mechanism(s) of Action of Amantadine relating to the Treatment of Fatigue in MS
The dopamine [DA] system: In 1968, a male patient with mild-to-moderate Parkinson’s Disease [PD] a disease characterized by selective loss of DA-secreting cells originating in substantia nigra, reported that, while taking amantadine for the prevention of influenza, noticed a remarkable remission of the PD symptoms, tremor and cogwheel rigidity. The symptoms re-appeared upon cessation of amantadine. L-Dopa, the metabolic precursor and substrate for the enzyme L-Dopa Decarboxylase [DDC] responsible for the synthesis of DA is still widely used for the treatment of PD. Studies in synaptic membrane preparations and in experimental animal models have consistently shown that amantadine also has the potential to increase the synthesis, turnover and synaptic release of DA [35-37]. Neuroimaging studies in patients with MSRF manifest both structural and functional impairments in brain structures such as striatum and prefrontal cortex that are heavily dependent on innervation by dopaminergic neurons. Moreover, a range of medications with the potential to stimulate the production of DA have been shown to improve connectivity between these regions leading to decreased fatigue [17].
NMDA receptor antagonists: In a ground-breaking clinical study, neurophysiological correlates of central fatigue were studied in patients with MS before and after treatment with amantadine compared to an equal number of healthy controls. The study made use of a standardized fatiguing repetitive tapping task and recording of an intra-cortical inhibitory phenomenon known as “the Cortical Silent Period [CSP]” by a distinct hand muscle acting as prime mover of the fatiguing indexthumb tapping task. At baseline, CSP was shorter in patients compared to controls. Chronic treatment with amantadine annulled these CSP duration differences and this was attributed to the restoration of physiological levels of intracortical inhibition. The authors suggest that the mechanism responsible related to amantadine’s NMDA receptor antagonist properties by which dysfunctional cortical GABA/glutamate ratios were redressed [15].
However, there is potential for an alternative mechanism involving interactions between NMDA receptor antagonists and the DA system via stimulation of DDC. Indeed, such a mechanism has been established using PET in both basal ganglia and cerebral cortical structures where neurologically-normal volunteers were administered 6-[18F] fluoro- L-Dopa, iv over 120 min prior to and 3 days following treatment with amantadine [100mg/d po]. DDC activities and fluxes via the enzyme pathway were increased following treatment with amantadine in basal ganglia [caudate nucleus (+12%), putamen (+28%), ventral striatum (+27%) as well as in frontal cortex (+9%). It was proposed that increased synthesis of DA resulting from increased DDC secondary to NMDA receptor antagonism by amantadine represents an important mechanism underlying the beneficial effects of amantadine [38].
The immune system: Based on studies in primary cultures of neurons, astroglia and microglia, it has been suggested that clinically-relevant concentrations of amantadine have the potential to exert neuroprotective actions resulting in a reduction of release of proinflammatory cytokines from activated microglia together with increased expression of the neurotrophic factor GDNF [39-41].
Interestingly, proinflammatory cytokines including tumour necrosis factor alpha [TNFα], Interferon gamma [IFNγ] and the interleukins IL-6 and IL-10 are reportedly increased in a range of disorders linked to fatigue including viral infections [42] some cancers [43] and in chronic fatigue syndrome [44]. Consequently, a study of cytokine levels in 37 MS patients with relapsing/remitting [n=29] or secondary progressive [n=8] disease age 41.0 ± 10.2 yrs. was initiated. Significant increases of serum TNFα mRNA were observed in patients with fatigue of FSS>4.0, n=26 or FSS>5.0, n=14 compared to MS patients without fatigue and FSS<4.0, n=11. No changes were noted for IFNγ or interleukins.
A range of medications are currently employed or under consideration for use in the treatment of fatigue in patients with MS. These agents include amantadine, the agent selected for assessment in the present study. Other agents [modafinil, pemoline, carnitine, aspirin, ondansetron, methylphenidate] were, in some trials, compared to amantadine in terms of efficacy and, in almost all cases in which it was studied, amantadine was compared to placebo. Results of the present investigation yielded an impressive number of published results based on 11 RCTs of amantadine and 6 systematic reviews with 2 associated meta-analyses of the findings which confirm, for the most part, that amantadine provides relief from fatigue in patients with either chronic persistent or relapsing-remitting MS.
An additional finding from one of the RCTs came in the form of a breakthrough in the search for the precise mechanism responsible for the benefits of amantadine for the treatment of MS-related fatigue. Making use of a novel standardized repetitive fatiguing task, it was determined that chronic treatment of MS patients with amantadine [200mg/d, 3 months] resulted in improvements in physiological levels of intracortical inhibition in motor cortex via normalization of GABA/glutamate balance leading to improvements in central fatigue scores in these patients.
Comparisons of efficacy conducted either as crossover or head-to-head designed trials provide evidence of the efficacy of amantadine not only compared to placebo but also, in the majority of cases, to alternative pharmacological approaches for the treatment of MS-related fatigue. These findings underpin the recommendations contained in The Clinical Practice Guidelines published by The Royal College of Physicians: Multiple Sclerosis [NICE, UK] and by The German Society of Multiple Sclerosis that amantadine is an effective pharmacological treatment for MS-related fatigue.
Citation: Pilling, Kerstin & Butterworth, RF. Amantadine for the treatment of fatigue in Multiple Sclerosis: Systematic Review and Summary of the Evidence Base. J Mult Scler (Foster City), 2021, 8(10), 272.
Received: 11-Sep-2021 Published: 28-Oct-2021, DOI: 10.35248/2376-0389.21.8.272
Copyright: © 2021 Pilling K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.