Research Article - (2019) Volume 8, Issue 1
Fibroblasts and platelet-rich plasma (PRP) used in regenerative medicine. This study evaluated whether fibroblast alone or in combination with PRP promote burn skin wound healing in vivo. Twenty CBA male mice were divided to 4 treatment groups: control (0.05 mL of phosphate-buffered saline, intradermal), PRP (0.05 mL of PRP, intradermal), fibroblasts/PRP (4x104 fibroblast in 0.05 mL PRP, intradermal), and fibroblasts (4x104 fibroblast in 0.05 mL PBS, intradermal), immediately after burning with a metal rod (100 °C for 40 s). The healing process was evaluated by gross examination throughout the experimental period on day 4, 11, 14 and 21, and histologic examination on day 21. Cytokines, growth factors and NO were determined in PRP by ELISA. The results indicated that the combination fibroblasts with PRP significantly promote wound healing (p ≤ 0.05). Histological study on day 21 revealed that fibroblast/PRP-treated wounds showed enhanced reepithelialization, sebaceous gland, hair follicles, and blood vessels formation. PRP contains wide-range of cytokines, growth factors, and NO. The current results demonstrated that fibroblasts in combination with PRP possesses beneficial burn skin wound healing.
<Intradermal injection, Burn wound, Fibroblasts, Platelet-rich plasma, Wound healing.
Skin wound healing is a coordinated process comprising an inflammatory reaction, angiogenesis and formation of extracellular matrix accompanied by scar tissue remodelling. Cellular participants as well as multiple growth factors and cytokines released by the cells at the wound site regulate these processes and finally result in wound closure. Deregulated healing processes may delay repair and may eventually lead to chronic wounds, such as those observed in lower extremity ulcers (venous, diabetic), and are often unresponsive to standard treatment [1-3]. A full-thickness burn wound as result of exposure to hot water or fire leads to loss of viable epidermis and dermis. Developed alterations in inflammation and granulation tissue formation are a mane differs during wound healing of burns [4].
Among the cells involved in skin wound healing, fibroblast important to the end state, because produced large amounts of molecules, which induced inflammation, and angiogenesis, include vascular endothelial growth factor (VEGF) production [5].
Cellular technologies often used for augment wound healing. So, early we established in mice with or without diabetes mellitus induced by streptozotocin, that therapy of burn skin wound by administration of bone marrow mesenchymal stem cells or conditioned medium from bone marrow mesenchymal stem cells possess to rapid skin epithelialization [6,7].
Platelet-rich plasma (PRP) used in regenerative medicine for promote tissue healing as alternative source of growth factors and cytokines which involved in damage tissue regeneration [8-10]. Platelets through hemostatic and production of cytokines and growth factor can influence at the wound healing [11]. The α granules of platelets contain numerous growth factors including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), VEGF, fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF1), and epidermal growth factor (EGF) and bioactive factors, such as nitrogen oxide (NO) [12,13].
Therefore, for searching capability of fibroblast alone or combined with platelet-rich plasma to promote burn skin wound healing was evaluated in vivo on a mouse model.
Animals
All animal experiments were carried out in full compliance with the standard international ethical guidelines (European Communities Council Directive 86/609/EEC). The study protocol was approved by the Local Institutional Ethics Committee of the Research Institute of Clinical and Experimental Lymрhology for the use of animals. A total number of 20 male CBA mice, weighing 20-25 g were obtained from the vivarium of the Institute of Physiology and Basic Medicine, Novosibirsk, Russia. The animal was housed 5 per cage in room maintained at 22 ± 0.5ºC and relative humidity of 65-70%, under a 12-hour light/dark cycle with free access to food and water. The animals were allowed to adapt to the laboratory for at least 2 hours before testing and were only used once. Experiments were performed during the light phase of the cycle (10:00-17:00). The animals were sacrificed under deep ether general anaesthesia. The protocol of anaesthesia, burn induction, post-burn care and sacrifice were identical for all animals.
Experimental Design
Male CBA mice were anesthetized with ketamine (15 mg/kg, Sigma-Aldrich) and shaved to remove all hair from the site of injury. One full thickness burn, having a circular diameter of 16 mm, was produced on the back of the mice by contact with a metal rod (100°C for 40 Sec). Animals were divided to 5 groups (n=5); group I: mice treated with phosphate-buffer saline (PBS; 0.05 mL, intracutaneous in 5 pointes around burned area) once immediately after burning (Day 0), group II: mice treated with human fibroblast cells (4x104 cells, intracutaneous in each from 5 pointe around burned area) once immediately after burning, group III: mice treated with PRP (0.05 mL, intracutaneous in 5 pointes around burned area) once immediately after burning, group IV: mice treated with PRP + human fibroblast (0.05 mL+4 × 104 cells, intracutaneous in each from 5 pointe around burned area) once immediately after burning. The wounds were observed daily. Estimates of wound area were calculated from the product of two mutually perpendicular perimeters. Wound area was measured using a caliper. Wound healing was quantitatively measured and calculated by the remaining wound area.
Isolations of human fibroblast cells from skin
Human skin specimens were obtained, with both informed donor consent and Human Research Ethics Committee approval of the Research Institute of Clinical and Experimental Lymphology Hospital, from donors. Skin specimens were placed in a large Petri dish and washed in PBS. Then specimens were cut to 2 pieces of 1 cm, then all pieces were placed in culture T-25 flask in culture medium containing Dulbecco’s modified Eagle’s medium (DMEM; Hyclone) supplemented with 10% heat-inactivated fetal caw serum (FCS, Hyclone), 80 μg/mL gentamycin (Gibco), and incubated in a humidified incubator at 37°C and 5% CO2 in air. The medium was exchanged every 2-3 days.
Preparation of platelet-rich plasma
Whole blood was collected from donors. Venous blood was aspirated using a syringe fitted with a 23-gauge scalpel needle into special PlasmoliftingTM (China) tube. Blood was centrifuged at 3200 g for 6 min (EBA200, Hettich, Germany), then platelet-rich plasma was collected and store -200C before used.
ELISA
The concentration of IL1β, IL6, IL8, IL10, TNFα, IFN-γ, Epo, PDGF-AB in PRP were measured using sandwich enzyme-linked immunosorbent assay (ELISA) kits (Vector-Best, Russia) and IGF1, TGFβ1 in PRP were measured using ELISA kits (Sigma-Aldrich, USA), according to the manufacturer’s instructions.
Nitrite Production
Nitrite was measured as an indicator of NO in PRP. NO inhibition assay was conducted using Griess reagent kit for nitrite determination (Molecular Probes). The amount of NO was calculated using a sodium nitrite standard curve.
Histopathological examination
A histological examination of the wound was performed 21 days after the treatment. The animals were sacrificed and tissue samples were bisected along the widest line of the wound, then fixed in 4% w/v neutral buffered paraformaldehyde for 48 hrs, dehydrate with gradient alcohol series, cleared in xylene and embedded in paraffin. Sections (5 μm) were obtained using Leitz microtome (Wetzlar, Germany) and were stained with haematoxylin and eosin (H&E). The slices were examined at the Optovar 1.25X under a light microscope Axio Observer Z1 Zeiss (Oberkochen, Germany) equipped with a digital camera (AxioCam ICc3).
Statistical analysis
Data were analyzed by the Statistica 10.0 for Windows. One-way analysis of variance (ANOVA) with a Bonferroni correction (Bonferroni post hoc test) was performed to analyze differences between groups. p values ≤ 0.05 were considered statistically significant. All results obtained are presented as the mean ± SD (standard deviation).
Platelet, cytokine, growth factor and NO in PRP
The number of platelets, levels of pro-inflammatory (IL1β, IL6, IL8, and TNFα), anti-inflammatory (IL4, IL10) cytokines, growth factors (Epo, PDGF-AB, and IGF-1), TGF-β1 and NO were all obtained at a higher concentration (Table 1).
| Parameters | Quantity |
|---|---|
| Platelets (× 104/mL) | 81.31 ± 15.71 |
| IL1ß (pg/mL) | 8.4 ± 1.5 |
| TNFa (pg/mL) | 9.8 ± 1.4 |
| IL4 (pg/mL) | 16.7 ± 3.5 |
| IL6 (pg/mL) | 8.6 ± 0.83 |
| IL8 (pg/mL) | 31.4 ± 6.9 |
| IL10 (pg/mL) | 2.5 ± 1.2 |
| Epo (pg/mL) | 984.8 ± 522.4 |
| PDGF-AB (pg/mL) | 797.2 ± 237.8 |
| IGF-1 (ng/mL) | 0.6 ± 0.1 |
| TGF-ß1 (pg/mL) | 48.2 ± 27.3 |
| NO (µM/mL) | 48.4 ± 25.3 |
PRP: Platelet-Rich Plasma; IL: Interleukin; TNFa: Tumor Necrosis Factor Alpha; Epo: Erythropoietin; PDGF-AB: PlateletDerived Growth Factor-AB; IGF-1: Insulin-Like Growth Factor-1; TGF-ß1: Transforming Growth Factor Beta1; NO: Nitrogen Oxide
Table 1. The average number of Platelets, levels of Cytokines, Growth Factors and NO in PRP (± Standard Deviation).
In vivo efficacy on a mouse burn wound model
2 shows full-thickness excisional wounds obtained immediately after burning on the back of male CBA and 4, 11, 14, and 21 days after treatment with PRP or Fb alone or in combination. At the beginning of the experiment skin lesion makes approximately 20.0 mm up to 10% of the total body surface and extending to all layers of skin, but not involving the muscular tissue. On Day 21 wound recovery equal to 74.19 ± 3.60%, 84.26 ± 4.80%, and 68.59 ± 8.43% was observed for PRPtreated, Fb/PRP-treated, and Fb-treated group of mice, respectively. Whereas, in control group (PBS-treated) wound recovery was observed in 59.91 ± 4.70% (Table 2). The combination of fibroblast with PRP possess to faster wound healing compare with control group and other treated group of mice (p ≤ 0.05).
| Groups | Day 0 | Day 4 (% wound recovery) | Day 11 (% wound recovery) | Day 14 (% wound recovery) | Day 21 (% wound recovery) |
|---|---|---|---|---|---|
| PBS-treated | 20.0 ± 0.9 | 19.0 ± 0.9 (4.91 ± 1.29%) | 16.4 ± 1.3 (18.0 ± 6.78%) | 12.4 ± 1.1 (37.84 ± 7.61%) | 8.0 ± 0.7 (59.91 ± 4.70%) |
| PRP-treated | 20.0 ± 0.9 | 19.9 ± 2.6 (1.11 ± 8.88%) | 11.8 ± 2.8a (41.03 ± 13.59%) | 7.0 ± 0.9b (64.84 ± 3.66%) | 5.2 ± 0.8c (74.19 ± 3.60%) |
| Fb/PRP-treated | 19.8 ± 0.8 | 18.7 ± 1.4 (5.31 ± 7.25%) | 12.6 ± 1.1 (36.41 ± 4.47%) | 7.0 ± 1.2b (64.65 ± 6.18%) | 3.1 ± 0.8b,d,e (84.26 ± 4.80%) |
| Fb-treated | 19.8 ± 0.8 | 18.8 ± 2.3 (4.72 ± 7.95%) | 13.5 ± 2.3 (31.95 ± 9.08%) | 10.0 ± 2.5 (49.66 ± 10.92%) | 6.2 ± 1.5 (68.59 ± 8.43%) |
PBS: Phosphate Buffered Solution; PRP: Platelet-Rich Plasma; Fb: Human Skin Fibroblast; astatistically differences with PBStreated group p = 0.05; bstatistically differences with PBS-treated group p = 0.001; cstatistically differences with PBS-treated group p = 0.00001; dstatistically differences with PRP-treated group p = 0.05; estatistically differences with Fb-treated group p = 0.05.
Table 2. Therapeutic effect of fibroblast, platelet-rich plasma, and combination of fibroblast with platelet-rich plasma on the wound healing in CBA male mice.
Histological findings on Day 21 burn wound healing
In control group, the formation of a thin layer of the epidermis is noted on most of the wound surface was observed (Figure 1a), but at the same time, there were areas of the wound surface without epidermis and keratinizing epithelium. Complete and well-organized epidermis, connective tissue contained blood vessels, sebaceous glands, and hair follicles in Fb-treated, PRPtreated and Fb/PRP-treated groups, especial in Fb/PRPtreated group (Figure 1b, 1c, and 1d).
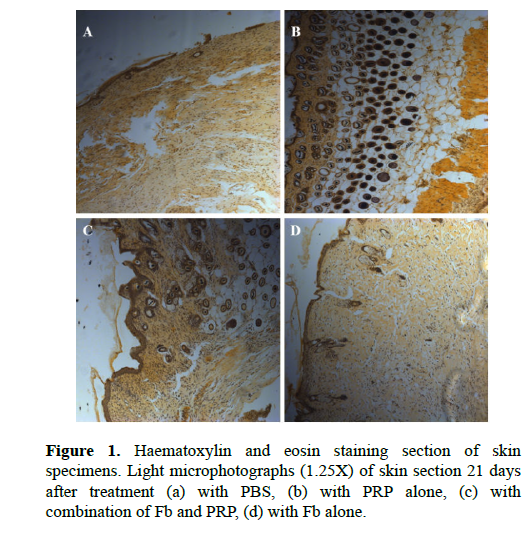
Figure 1. Haematoxylin and eosin staining section of skin specimens; Light microphotographs (1.25X) of skin section 21 days after treatment (a) with PBS, (b) with PRP alone, (c) with combination of Fb and PRP, (d) with Fb alone.
Wound healing is a complex process, which includes: coagulation, inflammation, migration/proliferation, and remodelling [10]. For good wound healing in burn skin lesion should be combination of reduction inflammation, forming of granulation tissue, high epithelial cell proliferation and neoangiogenesis.
Our present data demonstrated that injection of PRP alone or engraftment of fibroblast alone or with PRP intradermal around burn skin wound demonstrated a good re-epithelialization in CBA male mice.
Re-epithelialization is essential steps of wound healing, which protect from infection in wound. On Day 11 in PRP-treated, and Fb/PRP-treated groups, wound recovery was significantly higher (64% Vs. 37% in PBStreated group, p ≤ 0.05). On Day 21 in these groups wound recovery significantly increased and come up to 74% and 84%, PRP-treated and Fb/PRP treated groups, respectively.
Platelet-rich plasma are a source of many bioactive factors, include cytokines, growth factor, hormones, and other bioactive molecules [13,14]. We observed, that PRP-treated burn wound in CBA male mice showed faster healing compare PBS-treated group. This effect of PRP may be as acceleration of the damaged tissue regeneration [13-16]. PRP injection significantly increased angiogenesis, re-epithelialization, sebaceous gland reformation, and hair regrowth in burn wound.
Fibroblast are important at the end of wound healing, because may be as a source of bioactive molecules, and differentiation to myofibroblast, which is necessary to wound contract to close the wound [5,17,18]. Fibroblast in combination with PRP engraftment intradermal significantly accelerates wound healing compare with other groups of CBA male mice.
Microscopic examinations indicate, that PRP alone, Fb alone or in combination with PRP significantly promotes restoration of skin in burn wound area, compare with PBS-treated group. So, single intradermal engraftment of fibroblast alone or in combination with PRP around of burn lesion promote a significant to hair follicle, sebaceous gland, and blood vessels formation compare with PBS-treated group of CBA male mice on Day 21.
Data of ELISA indicates, that PRP contained a wide range of cytokines (IL1β, TNFα, IL4, IL6, IL8, IL10, TGF-β1), growth factors (Epo, PDGF-AB, IGF-1), and NO, which involved in inflammation, proliferation, and angiogenesis during wound healing [10,13,14,15,19,20]. So, productions of PDGF began after tissue damage and promote cellular reaction during wound healing. IGF involved in inflammatory and proliferative phase of wound healing. TGF-β1 promotes fibroblast and myofibroblast differentiation [15].
In our study, we demonstrated that treatment with RPR alone or in combination with fibroblast enhanced vessels formation, suggesting that PRP contains angiogenic growth factor. IL1β, TNFα, IL4, IL6, IL8, IL10, and NO may be involved in regulation of proliferation, differentiation and migration different cells type during wound healing into wound bed. These mechanisms are complex and interrelated with each other.
Obtained in our study accelerated burn wound healing after treatment with PRP, fibroblast alone or in combination is agreement with experimental and clinical outcomes. So, use for wound therapy fibroblasts (human fibroblast allografts) gives a good wound healing in patients with diabetic foot ulcers [17,18]. In rabbits nonhealing wound model intralesional PRP injection possess wound closure [13,20]. In dog skin wound model, PRP injections accelerated wound healing, markedly increased granulation tissue formation and angiogenesis, re-epithelialization [11]. Tissueengineering devices (artificial leather, tissue-engineering skin in combination with epidermal growth factor, or tissue-engineering skin in combination with the main growth factors for fibroblasts, or tissue-engineering skin with PRP) revealed the best efficacy in wound healing in mice by the combination of tissue-engineering skin with PRP [12].
It should be noted that this study had several limitations. In our study, we induced burn wound on normal skin, and no more that 10% of total body surface. We have no study of prolonged effect of PRP and fibroblast treatment. The number of platelets, and cytokines levels varied among the subjects.
The present study reported that intradermal engraftment of fibroblast with platelet-rich plasma was effective for burn skin wound healing in mice. Increased re-epithelialization, sebaceous gland, hair follicles, and blood vessels were obtained after fibroblast with platelet-rich plasma intradermal injection. This methods skin lesion therapy a useful alternative in the cutaneous wound healing.
We thank Ekaterina Lykova for assistance in preparing article.
There has been no financial support for this work.
Alexander Petrovich Lykov designed the study, contribute to the in vivo and in vitro study, contribute to the data entry and analyzed data, wrote the paper. Olga Vladimirovna Poveshchenko designed the study, read and approved the final manuscript. Natalia Anatollievna Bondarenko contribute to obtaining fibroblast, read and approved the final manuscript. Maria Alexandrovna Surovtseva contribute to preparing platelet rich plasma, read and approved the final manuscript.
The authors declare no potential conflicts of interests with respect to the authorship and/or publication of this paper.
Received: 14-Sep-2018
Copyright: © 2019 Alexander Petrovich Lykov et al. This is an open access paper distributed under the Creative Commons Attribution License. Journal of Biology and Today's World is published by Lexis Publisher; Journal p-ISSN 2476-5376; Journal e-ISSN 2322-3308.