Research Article - (2021) Volume 10, Issue 1
The plant species Plumbago zeylanica Linn. (Known vernacularly as Chitraka, Chitramulamu, Tellachitramulamu, Agnichela, Agnimaala by its trade or popular names of “Lead wort-white flowered” and “Ceylon Lead wort”) belongs to the family Plumbaginaceae, is distributed as a weed in throughout the tropical and subtropical countries of the world. The main objective of this study was to investigate the phytochemicasl, antioxidant and antimicrobial activities of the crude extract of Plumbago zeylanica leaves. Plumbago zeylanica was a rich source of secondary metabolites such as flavonoids, glycosides, cardiac glycosides, phenols, tannins, saponins, Steroids and quinones. It has prominent antioxidant activities. Among the selected pathogens highest zone of inhibition was observed by Clostridium septicum (10 mm) followed by Candida albicans (16 mm). The findings of this study support the view that P. zeylanica plant is a promising source of potential antioxidants and may be effective as preventive agent in the pathogenesis of some Ethno-Veterinary diseases.
Plumbago zeylanica • Phytochemical • Antioxidant activity • antimicrobial activity
Nature has been the source of therapeutic agents for treating human and livestock diseases since the dawn of civilization. Medicinal plants have a long history of use in the treatment of both human and animal diseases. A large number of modern drugs have been isolated from natural sources particularly from the plant world. The tribal communities still depends on medicinal plants for their first aid remedies to treat some simple ailments of livestock. Ethno-Veterinary Medicine (EVM) or Veterinary Anthropology refers to holistic and interdisciplinary study of traditional knowledge, skills, methods, practices and folk beliefs of the people about the health care, healthful husbandry and production of livestock [1]. The plant based traditional medicine systems continue to play an essential role in health care, with about 80% of the world’s farmers, shepherds and animal growers relying mainly on traditional medicines for treating routine maladies for their livestock [2,3]. Traditional veterinary medicine was experienced as early as 1800 BC at the time of King Hammurabi of Babylon who devised laws on veterinary fees and charged for treating animals. Many ethnoveterinary medicines were neglected due to the development of initial western drugs. Ethnoveterinary practices have gained tremendous importance for the last decade due to the discovery of some effective ethnoveterinary products [4]. Traditional veterinary medicines provide a cheap therapy and easy accessibility in comparison with western drugs [5]. Ethnoveterinary practices are more common in developing countries due to different socioeconomic factors. Traditional medicine is a part of the indigenous knowledge system of the people all over the world. According to the World Health Organization (WHO), at least 80% of the people in developing countries depend on indigenous practices for treatment of various diseases of both human being and their animals [6]. The medicinal plants are also used extensively and quite effectively for primary health care treatment of the domestic animals [7]. Livestock keeping is one of the most important economic sources of rural community. The farmers and nomadic people not only depend on plants to get fodder for their animals but also use different medicinal plants to treat various animal diseases. The majority of the people interviewed using ethnoveterinary plants have got this knowledge from their forefathers while some have learned from the other people. The majority of the farmers and nomadic pastoralists were not very well off and heavily dependent on medicinal plants due to their unaffordable potential of using modern veterinary drugs for their animals treatment. Generally freshly collected plants or plant parts are used for treatment. Most commonly used parts were seeds, leaves, whole plant and fruit but in many cases roots, young twigs, bark powder, seed oil were also used for treatment. Single plant is used to treat some diseases as well as combination of two or more plants is also used to treat many other diseases.
Collection of plants
Plumbago zeylanica L. (Plumbaginaceae) commonly called as Doctorbush. Plumbago is a genus of 15-20 species of Angiosperms in the family Plumbaginaceae. The family Plumbaginaceae consists of 10 genera and 280 species. The genus Plumbago includes 3 species, namely Plumbago indica L. (P. rosea L.), P. capensis L., and P. zeylanica L., which are distributed in several parts of India. Among these species Plumbago zeylanica grows all districts of plains in Tamilnadu, Andra Pradesh, Karnataka and Kerala [8].
Plumbago zeylanica commonly called as Ceylon Leadwort is a semi climbing sub shrub that grows throughout Asia, Australia, Africa and Ceylon and widely used in ethnomedicine. It is used in indigenous system of medicine, and commonly known as Chitthra mulam. It is branched evergreen shrub growing up to 2 meters. The leaves are dark-green, ovate 30 cm long and 15 cm wide. The flowers are white in thick racemes, individuals around 1 cm across, flowering throughout the year.
Preparation of extracts
Collected plant leaves were dried at room temperature and ground to make fine powder. 20 g of plant powder was well dissolved in 100 ml of solvents (Hexane, Ethyl acetate and Ethanol) (ratio 1:5). The suspension was filtered by using Filter paper of pore size 0.2 μm. The filtrate was then air dried and extracts were collected in sterile vials for further use.
Maceration
4 Kg of dried leaves were macerated using 8.6 L methanol in 1 L conical flasks at room temperature for 24 hours. Then the extracts obtained were filtered using Whatman filter paper to obtain methanol extract. The residue left was again subjected to second successive extraction with methanol, following previously mentioned procedure to get the second methanol extract. Then both extracts were studied for their TLC profiles and owing to their similar TLC pattern they were mixed. Thus obtained methanolic extract was concentrated in rotary flash evaporator and dried in a vacuum oven so as to obtain thick, viscous mass. Then the yield value was calculated and the concentrated methanolic extract was subjected to the successive extraction using hexane, chloroform and methanol to obtain hexane, chloroform and methanol soluble fractions. Finally, yield value for each extract was calculated.
Phytochemical tests
The phytochemical test of these extracts was performed using the method adopted by Harborne and Sofowora.
Test for Carbohydrates (Molisch’s Test) to 2 ml of plant extract, 1 ml of Molisch’s reagent and a few drops of concentrated sulfuric acid were added. Presence of purple or reddish color indicates the presence of carbohydrates.
Test for Tannins (Ferric Chloride Test) to 1 ml of plant extract, 2 ml of 5% ferric chloride was added. Formation of dark blue or greenish black indicates the presence of tannins.
Test for Saponins (Frothe’s Test) to 2 ml of plant extract, 2 ml of distilled water was added and shaken in a graduated cylinder for 15 minutes lengthwise. Formation of a 1 cm layer of foam indicates the presence of saponins.
Test for Flavonoids (Shinoda Test) to 2 ml of plant extract, 1 ml of 2 N sodium hydroxide was added. Presence of yellow color indicates the presence of flavonoids.
Test for Alkaloids (Mayer’s Test) to 2 ml of plant extract, 2 ml of concentrated hydrochloric acid was added. Then a few drops of Mayer’s reagent were added. The presence of green color or white precipitate indicates the presence of alkaloids.
Test for Quinones to 1 ml of extract, 1 ml of concentrated sulfuric acid was added. Formation of red color indicates presence of Quinones.
Test for Glycosides (Molisch’s Test) to 2 ml of plant extract, 3 ml of chloroforms and 10% ammonia solution was added. Formation of pink color indicates presence of glycosides.
Test for Cardiac Glycosides (Keller-Kiliani Test) to 0.5 ml of extract, 2 ml of glacial acetic acid and a few drops of 5% ferric chloride were added. This was under layered with 1 ml of concentrated sulfuric acid. The formation of brown ring at the interface indicates presence of cardiac glycosides.
Test for Terpenoids (Salkowski Test) to 0.5 ml of extract, 2 ml of chloroform was added and concentrated sulfuric acid is added carefully. Formation of red brown color at the interface indicates presence of terpenoids.
Test for Phenols (Ferric Chloride Test) to 1 ml of the extract, 2 ml of distilled water followed by a few drops of 10% ferric chloride was added. Formation of blue or green color indicates presence of phenols.
Test for Proteins: To the 0.5 ml of extract, 0.5 ml of extract, 2 ml of 40% sodium hydroxide and 1 ml 1% CuSO4 were added formation of pink colour indicates presence of proteins.
Antioxidant activity
DPPH assay: The antioxidant activity was determined by DPPH assay as described earlier with some modifications. From the stock solution different concentrations of extract (100 μg-600 μg/ml) were prepared. 200 μl of each concentration was mixed with 3.8 ml DPPH solution and incubated in the dark at room temperature for 60 min. Absorbance of the mixture was then measured at 517 nm controls and Vitamin E was used as a positive. Scavenging ability of the sample to DPPH radical was determined according to the following equation.

Ferric reducing power assay: Ferric reducing/antioxidant power (FRAP) was determined following the method as described earlier. Briefly, 100 μl of each concentration of the extracts (100-600 μg/ml) was mixed with 2.5 ml of 200 mM phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide and incubated at 50°C for 20 min. After this, 2.5 ml of 10% trichloroacetic acid was added and the tubes were centrifuged at 10,000 rpm for 10 min. Five milliliters of the upper layer of the solution was mixed with 5.0 ml of distilled water and 1 ml of 0.1% ferric chloride and the absorbance of the reaction mixtures was measured at 700 nm. The final results were expressed as mg ascorbic acid equivalent/g of dry weight.
FRAP: FRAP values were higher in methanol extracted samples compared to hexane and chloroform extraction. This showed that methanol extraction was more efficient in extracting antioxidants in plant materials compared to water.
Antimicrobial activity: The most common diseases observed in animals are Aspergillosis, Candidiasis, Salmonellosis, Brucellosis etc. Due to the presence of pathogenic activity in the veterinary the below organisms are selected for the study. Five different strains of microorganisms were used in the screening listed in Table 1.
| Organism | NCIM Accession No |
|---|---|
| Brucella abortus | 5282 |
| Clostridium septicum | 2132 |
| Aspergillus fumigatus | 1272 |
| Candida albicans | 3102 |
| Salmonella enterica | 1164 (MTCC No) |
Table 1: List of equipments/insruments used in the study.
Antibacterial activity by agar well diffusion method
The bacterial and fungal cultures were grown over night at 37°C and 28°C for testing antimicrobial activity. Nutrient agar medium and Potato dextrose agar medium were distributed in 100 ml conical flasks separately and were sterilized in an autoclave at 121°C, 15 Lbp for 15 min; the media were poured into sterilized petri plates. The bacterial and fungal Inoculum was spread over the surface of agar plates with sterile glass spreader. Four wells for antibacterial and antifungal were made at equal distance using sterile cork borer.
Antibacterial activity was recorded when the zone of inhibition was greater than 8 mm. Studies were performed in triplicates and the mean value was calculated. For control antibiotic streptomycin was used for standardized.
Yield value determination
Yield value of each extracts was calculated as below
For methanolic Soxhlet extraction
Yield Value=(4 g/40 g) × 100%=10%
For methanolic macerated extract
Yield Value=(120 g/ 4000 g)=3%
Then methanolic extract was again successively extracted using hexane, chloroform and methanol as a solvent and the respective yields were calculated as shown in Tables 2 and 3.
| S. No | Extracts | Soxhlet Extraction |
|---|---|---|
| 1 | Hexane | 31.50% |
| 2 | Chloroform | 18.75% |
| 3 | Methanol | 50% |
Table 2: Yield values of different extraction process.
| S. No | Phytochemical test | Result |
|---|---|---|
| 1 | Alkaloids | - |
| 2 | Flavanoids | + |
| 3 | Glycosides | + |
| 4 | Cardiac glycosides | + |
| 5 | Tanins | +/- |
| 6 | Phenols | + |
| 7 | Steroids | + |
| 8 | Quniones | + |
| 9 | Proteins | - |
| 10 | Carbohydrates | - |
| 11 | Saponins | ++ |
Table 3: Preliminary phytochemical analysis of Plumbago zeylanica.
Phytochemical analysis
The results of preliminary phytochemical analysis of Plumbago zeylanica were given in the Table 3. Plumbago zeylanica was a rich source of secondary metabolites such as flavonoids, glycosides, cardiac glycosides, phenols, tannins, saponins, Steroids and quinones. In the present study, plant extractives were analyzed to estimate the percentage yield of individual extracts and found that, the yield was abundant in methanol rather than chloroform and hexane. Due to the high polarity of methanol most of the chemical constituents of extracts would be dissolved in it and thus percentage yield was increased tremendously than other solvents. This finding supports the earlier reports.
Flavonoids consist of a large group of polyphenolic compounds having benzo-γ-pyrone structure widely spread in plants. These are known to synthesize by plant in response to microbial infection and present in all plant parts. They are divided into a variety of classes such as flavons (flavon, epigenin and leutiolin) Flavonoles (querecetin, kempferol, myricetin), flavonones (flavonone, hesperrtin, naringenin) among which, flavonols are the most abundant flavonoids in food. These are the major colouring compounds of flowering plants. Flavonoids in food are responsible for colour, taste prevention of fat oxidation. Being phytochemicals, these cannot be synthesized by humans and animals.
Several studies suggested protective effects of flavonoids against bacterial, viral, cardiovascular diseases, cancer and other age related diseases. These act as secondary antioxidant defence system in plant tissues exposed to different biotic and abiotic stress. Their functional hydroxyl group is responsible for scavenging free radicals and chelating metal ions. Mechanism of antioxidant action can induce suppression of ROS formation (either by enzyme inhibition or metal chelation), scavenging ROS, upregulation or protection of ROS.
Some of the important enzymes involved in ROS generation are inhibited by flavonoids are monooxygenase, glutathione S transferase, mitochondrial succinoxidase, NADH oxidase etc. They protect lipid against oxidative damage. In addition there hepatoprotective, anti-inflammatory, anti-cancer, anti-bacterial, and anti-viral activities have been also reported.
Plant steroids are a distinctive class of phytoconstituents found throughout the animal and plant kingdom, a specific class of steroids, glucocorticoids are widely used for the suppression of inflammation in chronic inflammatory diseases which are associated with increased expression of inflammation genes by binding to glucocorticoid receptors on multiple signaling pathways. However, some adverse effects are also associated with their prolong use such as immunosuppression, hypertension, osteoporosis and metabolic disturbance.
Saponins are high molecular weight compounds in which a sugar molecule is combined with triterpene or steroid aglycon, so there are two major groups of saponins; triterpene saponins and steroid saponins. These are therapeutically important as they show hypolipidemie and anticancer activity of cardiac glycosides.
Tannins are phenolie compounds of high molecular weight soluble in water and alcohol and found in root, bark, stem and other layers of plant tissues, due to presence of phenolic groups these are used as antiseptic. In ayurvedic medicine system, tannin rich plant based formulations are used to treat leucorrhoea, rhinnorhoea and diarrhea.
Cardiac glycosides are the compounds used to treat congestive heart failure and cardiac arrhythmea. These compounds work by inhibiting the Na+/K+ pump.
Antioxidant activity
Antioxidants are the substances that neutralize free radicals or their actions. Reactive Oxygen Species (ROS), as well as reactive nitrogen species (RNS), are products of normal cellular metabolism and they are well recognized for playing a dual role as both deleterious and beneficial species, since they can be either harmful or beneficial to living systems [8]. Antioxidants are tremendously important substances which possess the ability to protect the body from damage caused by free radical induced oxidative stress.
The medicinal value of the plants lies in some chemically active substances that produce a definite physiological action on the human body. The most important of these bioactive constituents of plants are alkaloids, tannins, flavonoids and phenolic compounds. Antioxidants are vital substances which possess the ability to protect the human body from damage caused by free radical induced oxidative stress.
DPPH
The antioxidant activity of plant extract was determined using a methanol solution of DPPH reagent. The antioxidant activity was expressed in terms of percentage of inhibition (%) (Figure 1). The effect of antioxidants on DPPH is thought to be due to their hydrogen donating ability. The IC50 value thus obtained was 2.177. Relatively higher concentrations of the sample showed higher antioxidant activity compared to low and moderate concentrations of the fractions. Present results on antioxidant potential of Butea monosperma corroborate with the findings Rajakrishnan, R [9].
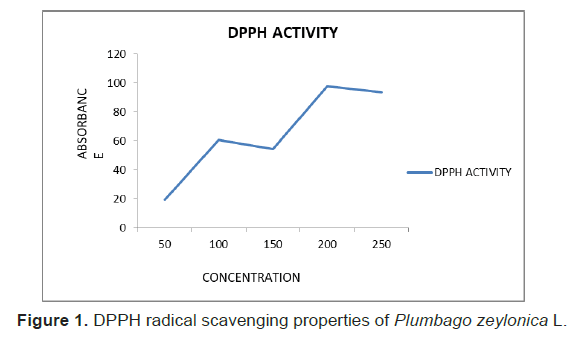
Figure 1: DPPH radical scavenging properties of Plumbago zeylonica L.
Ferric reducing antioxidant power (frap) assay
FRAP values were higher extracted methanol samples (67.84 ± 0.01) compared to in chloroform (65.58 ± 0.01) and hexane (53.82 ± 0.06) extraction.
Reducing power assay
The antioxidant activity was expressed in terms of percentage of inhibition (%). Reducing power assay chloroform and hexane (72.01%) followed by methanol (71.91%).
Total antioxidant activity
The Total antioxidant activity of plant extract was determined using a methanol solution. The Total antioxidant activity was expressed in terms of percentage of inhibition (%) (Figure 2). The IC50 value thus obtained was 1.77.
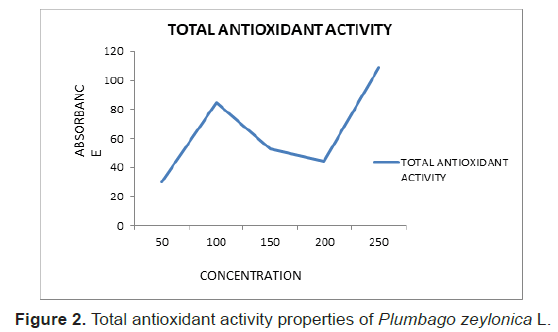
Figure 2: Total antioxidant activity properties of Plumbago zeylonica L.
Antimicrobial studies
Due to the failure of synthetic drugs, adverse side effects of antibiotics and antibiotic resistance of the microorganisms led to the development of new plant derived antibiotic without any side effects.
The microorganisms used in the study are clinically important pathogens which are causing several infections in animals. Among the medicinal plant experimental data only methanol extract exhibited moderate to high inhibition zones (activity) against all the microflora screened, Hexane and Chloroform extracts showed only mild to no activity, hence only methanol extract reports was analyzed. The antimicrobial activities of the extract against clinically isolated microbial strains were represented in Table 4. Highest zone of inhibition was observed by Clostridium septicum (10 mm) followed by Candida albicans (16 mm), Brucella abortus (15 mm), Aspergillus fumigates (14 mm) and Salmonella enteric (13 mm).
| S.No. | Organism | 100mg/ml | 50mg/ml | 25mg/ml | MIC | Standard | Control |
|---|---|---|---|---|---|---|---|
| 1. | Brucella abortus | 15 | 13 | 11 | 25 | 37 | 6 |
| 2. | Clostridium septicum | 17 | 12 | 11 | 25 | 37 | 6 |
| 3. | Aspergillus fumigatus | 14 | 12 | 11 | 25 | 36 | 6 |
| 4. | Candida albicans | 16 | 14 | 12 | 20 | 34 | 6 |
| 5. | Salmonella enterica | 13 | 12 | 10 | 25 | 36 | 6 |
6 mm borer size; 25 µl per well; Standard: Ciprofloxacin/ Fluconazole; Control: DMSO
Table 4: Antimicrobial activity of methanol extract of Plumbago zeylonica L.
Antimicrobial activities of Plumbago species have been reported by many workers, Ibrahim et al. evaluated the antibacterial activity using the methanolic extracts of P. indica against S. aureus, S. typhi, S. dysenteriae, B. cereus, P. aeruginosa, S. sonnei, V. cholera and E. coli [10]. The highest inhibition was observed against S. aureus, E. coli and S. typhi compared to other pathogens ranged from 15-27 mm.
Antibacterial activity of methanolic and chloroform extracts of P. zeylanica against five different organisms viz., S. pyogenes, S. aureus, Bacillus sp., P. aeruginosa and E. coli were studied using disc diffusion method. The methanolic extracts were more active against all the tested organisms [11].
Rahman and Anwar studied the antimicrobial activities of ethanolic extracts of P. zeylanica root against 11 human pathogenic bacteria and 6 phytopathogenic fungi using disc diffusion method [12]. V. cholerae was found to be the most sensitive. Wang and Huang revealed the anti-H. pylori activity of ethanolic, ethyl acetate, acetone and aqueous extracts of P. zeylanica by Paiva et al. evaluated the antimicrobial activity in the plumbagin isolated from the chloroform extract of P. scandens against S. aureus, P. aeruginosa, B. subtilis, P. vulgaris and against the yeast C. albicans [13,14]. Parekh et al. studied the antibacterial potential of P. zeylanica using agar disc diffusion method and agar well diffusion method against five bacterial strains viz., B. cereus, S. aureus, K. pneumoniae, E. coli and P. pseudoalcaligenes [15]. Preliminary screening revealed that methanolic extracts were more potent than the aqueous extracts.
Except Wang and Hang observation, all others were recorded the highest frequency of antibacterial activity in the methanolic extracts of Plumbago species (Table 4) [14].
Similar to that in the present study also we observed the high frequency of antibacterial activity in the methanolic extracts of Plumbago zeylonica. The phenolic compounds are known for their antimicrobial properties. The significance of these compounds is that these can substitute long term antibiotic therapy. The antibacterial action of various aerial parts extracts of Plumbago zeylonica may indicate their potential as antibacterial herbal remedies. Further work is needed to locate the active principle from the various extracts and their phyto pharmaceutical studies. Research into the effects of local medicinal plants is expected to boost the use of these plants in the therapy against disease caused by the test bacterial species and other microorganisms.
Citation: Usha SK, et al. Studies on Plumbago zeylanica: An Ethnoveterinary Plant. J Biol Today's World, 2021, 10(1), 001-004.
Received: 15-Dec-2020 Published: 05-Jan-2021, DOI: 10.35248/2322-3308.21.10.003
Copyright: © 2021 Usha SK et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.