Research Article - (2023) Volume 12, Issue 3
Background: Intravenous Immunoglobulin (IVIG) resistance prediction is a main topic of interest in Kawasaki disease (KD). In this study, we examined the value of Serum Amyloid A (SAA) for predicting IVIG resistance in KD patients.
Methods: 409 KD patients were retrospectively enrolled and divided into IVIG responsive (n=366) and IVIG resistant (n=43) groups according to the definition of IVIG resistance. Serum SAA levels prior to IVIG therapy were measured at the acute phase. Demographic, echocardiography, and laboratory data were also retrospectively analyzed.
Results: Levels of SAA, total bilirubin, C-reactive protein, neutrophils, alanine aminotransferase, aspartate aminotransferase, interleukin-6 and procalcitonin were significantly higher in the IVIG resistant group than in the IVIG responsive group (P<0.05), but the lymphocytes, platelets, serum sodium level and duration of fever before IVIG therapy were significantly lower (P<0.05). Multiple logistic regression analysis showed SAA (P=0.008), neutrophils (P<0.001), total bilirubin (P=0.001), platelets (P=0.004) and serum sodium(P=0.019) were independent predictors of IVIG resistance. The best cut-off value of SAA for IVIG resistance prediction was 252.45 mg/L, with a corresponding sensitivity of 69.8% and a specificity of 54.4%. Based on ROC curve analyses, The AUC of combined detection with these five indicators was 0.800; the sensitivity was 69.8%, and specificity was 76.2%.
Conclusions: SAA may be a candidate biomarker for predicting IVIG responsiveness to KD, Combined detection of SAA, total bilirubin, neutrophils, platelets and sodium is superior to that of any single indicator for predicting IVIG resistance in KD. Its use in clinical practice may help in treatment decisions.
Intravenous immunoglobulin • Serum amyloid • Resistance • Hemophagocytic syndrome • Septic lesions.
Kawasaki Disease (KD) is an acute self-limiting vasculitis that predominantly occurs in children younger than 5 years of age. Intravenous Immunoglobulin (IVIG) is the most effective initial treatment. However approximately 10-20% of patients do not respond to initial IVIG treatment [1]. These patients are at high risk of developing Coronary Artery Lesions (CALs) [2], the most serious complication and thrombotic occlusion is known to account for the sudden death in some KD patients [3]. The mechanism of IVIG in KD is still not completely understood, and IVIG resistance have not been identified [4,5]. Early prediction of IVIG resistance is paramount in KD, as such patients might benefit from early-intensification therapy and reduce the risk of CALs.
There are several scoring systems for predicting IVIG resistance, although these scoring systems are well utilized in Japan, they do not perform well in the other populations, which may be attributable to ethnic and other differences [6-11]. There were also some studies on IVIG resistance prediction models in China but some models had low sensitivity and specificity [12]. Some single biomarker such as the PTX3 level, procalcitonin, soluble CD163 and free carnitine can act as strong and sensitive predictors of IVIG unresponsiveness [13-15]. However, using common laboratory alone and clinical features is not sufficient to predict IVIG resistance [16,17].
Serum Amyloid A (SAA) is a highly conserved acute-phase protein. Similar to other acute-phase protein, C- Reactive Protein (CRP), SAA can be used as a diagnostic, prognostic, or therapeutic follow-up marker for many diseases [18-20]. Currently, SAA is frequently used as an indicator for monitoring inflammation and predicting prognosis in clinical practice. SAA is significantly upregulated in acute and chronic inflammatory conditions (such as trauma, infection, and ischemia), in response to the elevation of the Inflammatory Interleukin-6 (IL-6) and Tumor Necrosis Factor (TNF)-a during the acute-phase response [21]. Moreover, SAA levels rise in KD and their levels are predictive of CALs [22].
Nevertheless, few studies have investigated the possible link between SAA levels and IVIG resistance in KD. For this study, we hypothesized that SAA is useful for predicting IVIG resistance in KD patients and may be conveniently used by clinicians to identify IVIG resistance in these patients.
Patient selection
We searched the electronic medical records of Anhui Provincial Children’s Hospital to identify all patients admitted with a first diagnosis of KD from January 2019 to October 2022. Inclusion criteria were as follows: children hospitalized with complete KD or incomplete KD by American Heart Association criteria; IVIG and aspirin were routinely administered in accordance with guideline [1]. Exclusion criteria include the following: non-standard IVIG treatment; use of corticosteroids or other immunosuppressive drugs before the initial IVIG treatment or at the same time as initial IVIG treatment; presence of other serious complications such as macrophage activation syndrome, shock, severe infection, septic lesions, multiorgan dysfunction, hemophagocytic syndrome and clinical data during hospitalization missing.
All patients were received IVIG 2 g/kg as single dose with aspirin 30-50 mg/kg as recommended in guidelines [1]. After defervescence for 48-72h, a tapered dose of aspirin (3-5 mg/kg/day) was administered for 6-8 weeks. If patients had CALs, aspirin was continued until there was no evidence of the lesions. A patient was considered afebrile when body temperature remained below 37.5° for more than 24h. The definition of IVIG-resistance is determined by body temperature that is not resolved within 36 hrs after completion of IVIG infusion. This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Anhui Provincial Children’s Hospital (Approval Number EYLL-2022-022). The study protocol was explained to the participants and their parents and written informed consent was obtained.
Clinical and laboratory data collection
Data on baseline characteristics and laboratory test results during hospitalization were collected from the hospital records. Demographic data included gender, weight, age at KD diagnosis, incomplete Kawasaki disease, and clinical features (rash, conjunctivitis, oral changes, extremity changes, cervical lymphadenopathy). Laboratory test results included white blood cell count, hemoglobin, neutrophils, lymphocytes, platelets, SAA, CRP, erythrocyte sedimentation rate, serum albumin, aspartate transaminase, alanine aminotransferase, total bilirubin, serum sodium, interleukin-6 and procalcitonin. All blood samples were collected in the morning within 24 hrs of admission prior to initial use of IVIG. If multiple laboratory values were available, the value closest to the time of diagnosis and prior to IVIG administration was recorded. Blood samples were collected in heparin anticoagulant tubes and centrifuged at 1,000 g for 5 mins to separate serum. SAA levels were measured by the latex-enhanced immunoturbidimetric method with an automatic biochemical analyzer (Hitachi-7600). The reference value of SAA ranges from 0 to 10.00 mg/L.
Echocardiogram measurements
Data on cardiac outcomes were also collected from hospital records from color Doppler ultrasound examinations. CALs were defined on normalized dimensions for the body surface area as Z scores ≥ 2.5 of coronary arteries on the basis of maximal internal diameters of the right coronary artery, left anterior descending artery or left circumflex coronary artery [2]. Body surface area and Z scores were calculated using the Kobayashi equations [23].
Statistical methods
Data analysis was performed with SPSS 26.0 (IBM Corp, Armonk, NY, USA). Quantitative data are presented as the median with the 25th and 75th percentiles (Interquartile Range (IQR)) in square brackets, while qualitative data are expressed as n/% as appropriate. The Shapiro-Wilk test and homogeneity test of variance were used to confirm that quantitative data from different groups showed a normal distribution and met the homogeneity of variance. The chi-square test and unpaired Student’s test/Mann-Whitney U test were applied to compare demographic characteristics, clinical manifestations, and laboratory data between groups. Crucial indicators from univariate analysis were then subjected to multivariate logistic regression analysis to identify independent predictors of IVIG resistance. To compare the power of candidates to predict IVIG unresponsiveness, cut-off values based on the maximum value of the youden index according to sensitivity and specificity, were selected on the basis of a Receiver Operating Characteristic (ROC) curve. A value of P<0.05 was accepted as statistically significant.
A total of 497 consecutive KD patients were eligible, 88 were excluded from analysis according to the inclusion and exclusion criteria as follows: did not receive IVIG treatment prior to 10 days from fever onset (n=16), received steroids as initial therapy (n=33), had infectious diseases or immune diseases (n=10), did not have complete laboratory data (n=15), diagnosed but not treated with IVIG (n=6) and received IVIG at another hospital (n=8). The final analysis included 409 KD patients, 43 of whom (10.5%) showed IVIG resistance (Figure 1).
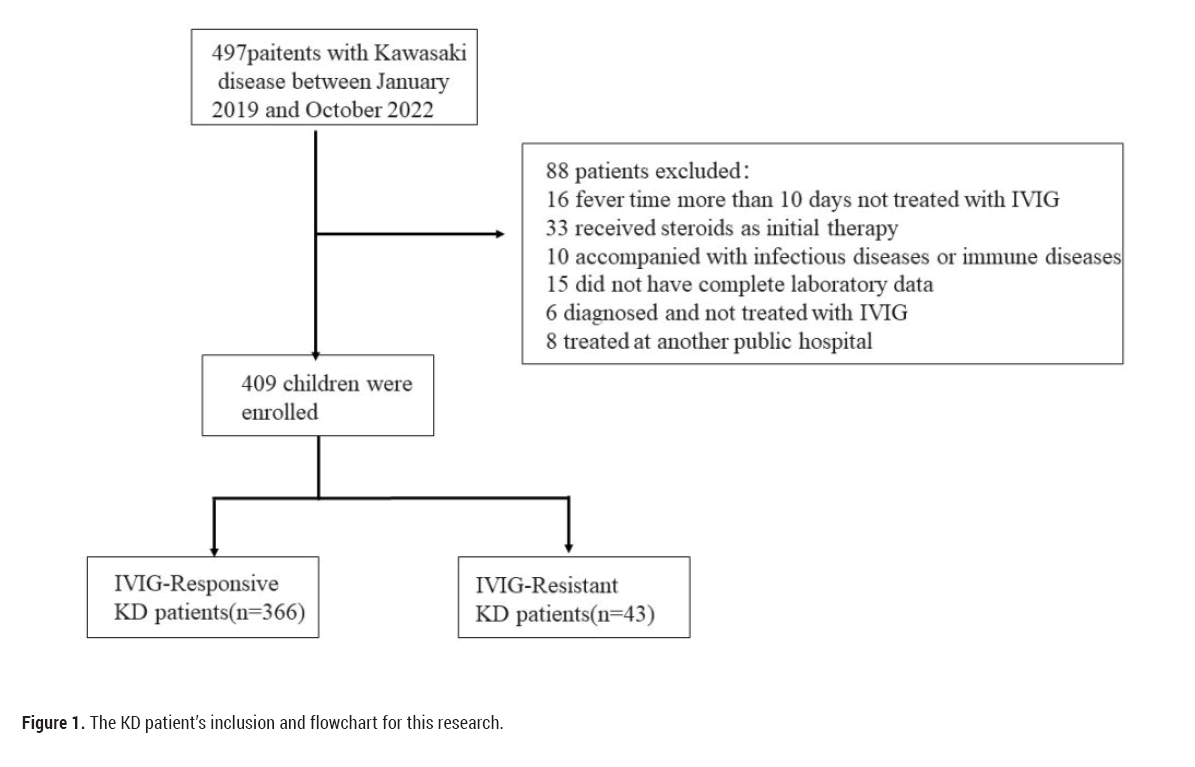
Figure 1: The KD patient’s inclusion and flowchart for this research.
As indicated in Table 1, there was no significant difference between the two groups regarding age, gender ratio or body weight. The fever duration before initial IVIG treatment was significantly shorter in the IVIG resistant group than in the IVIG responsive group (P<0.05). Regarding clinical manifestations, there were no significant differences in fever, conjunctivitis, cervical lymphadenopathy, rash, oedema of extremities, mucosal changes and the incidence of CALs between the groups. However, the mean (IQR) SAA level in the IVIG resistance group was significantly higher than that in the IVIG responsive group (380.00 [204.40-547.25] vs. 230.85 [105.40-490.00] mg/L; P=0.008), Significant differences were also found in other parameters, including neutrophils, alanine aminotransferase, alanine aminotransferase, total bilirubin, lymphocytes, platelets, serum sodium, IL-6 and procalcitonin.
| Parameters | Total (n=409) | IVIG-reply (n=366) | IVIG-resistance (n=43) | T/z/x2 | P-value |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, months, Median (IQR) | 25.00 (12.00,36.00) | 25.00 (12.00,36.00) | 24.00 (12.00,36.00) | -0.658 | 0.511 |
| Male, n (%) | 258 (63.10) | 228 (62.30) | 30 (69.80) | 0.923 | 0.405 |
| Weight, kg, Median (IQR) | 12.50 (10.00,15.40) | 12.50 (10.00,16.00) | 12.00 (9.75,14.50) | -1.047 | 0.295 |
| Clinical parameters | |||||
| Fever pre-IVIGs, d | 6.00 (5.00,7.00) | 6.00 (5.00,7.00) | 5.00 (4.00,6.00) | -2.653 | 0.01 |
| iKD n (%) | 31 (7.60) | 26 (7.10) | 5 (11.60) | 0.571 | 0.45 |
| Conjunctivitis | 37 (9.04) | 33 (9.01) | 4 (9.30) | 0 | 1 |
| Cervica lymphadenopathy, n (%) | 53 (9.01) | 48 (13.11) | 5 (9.01) | 0.075 | 0.784 |
| Rash, n (%) | 58 (14.18) | 51 (13.93) | 7 (16.27) | 0.174 | 0.677 |
| Erythema/edema of extremities, n (%) | 150 (9.01) | 132 (36.06) | 18 (41.86) | 0.556 | 0.456 |
| Mucosa changes, n (%) | 83 (36.67) | 70 (19.12) | 13 (30.23) | 2.935 | 0.087 |
| CALs, n (%) | 47 (11.49) | 39 (10.65) | 8 (18.60) | 1.673 | 0.196 |
| Laboratory parameters | |||||
| WBC, × 109/L, Median (IQR) | 14.49 (11.32,18.19) | 14.39 (11.17,17.91) | 15.49 (12.28,19.23) | -1.651 | 0.099 |
| Neutrophils, × 109/L, Median (IQR) | 9.37 (6.74,12.42) | 8.91 (6.65,12.03) | 12.00 (9.23,16.17) | -3.935 | <0.01 |
| Lymphocytes, × 109/L, Median (IQR) | 3.33 (2.05,5.04) | 3.43 (2.18,5.23) | 2.29 (1.39,3.17) | -4.155 | <0.01 |
| Platelets, × 1012/L, Median (IQR) | 346.00 (267.50,431.50) | 349.00 (276.00,440.00) | 279.00 (229.50,376.00) | -3.063 | 0.002 |
| hemoglobin, g/L, Median (IQR) | 108.00 (101.00,116.00) | 108.00 (102.00,115.00) | 108.00 (100.00,117.00) | -0.465 | 0.642 |
| SAA, mg/L, Median (IQR) | 239.90 (111.90,506.23) | 230.85 (105.40,490.00) | 380.00 (204.40,547.25) | -2.637 | 0.008 |
| CRP, mg/L, Median (IQR) | 67.90 (43.85,104.05) | 65.38 (41.80,102.10) | 99.60 (80.90,127.75) | -4.455 | <0.01 |
| ESR, mm/h Median (IQR) | 56.00 (34.00,78.00) | 56.00 (35.00,78.00) | 65.00 (29.50,78.50) | 0.259 | 0.796 |
| ALT, µ/L, Median (IQR) | 25.00 (14.60,63.30) | 24.00 (14.00,55.50) | 44.10 (19.65,136.00) | -2.819 | 0.005 |
| AST, µ/L, Median (IQR) | 28.50 (22.60,44.00) | 28.00 (22.00,41.00) | 34.00 (24.45,109.20) | -2.401 | 0.016 |
| ALB, g/L, Median (IQR) | 37.30 (34.45,40.10) | 37.40 (34.70,40.10) | 36.90 (31.25,55.50) | -1.341 | 0.18 |
| TBIL, µmol/L, Median (IQR) | 6.80 (4.80,10.15) | 6.60 (4.70,9.60) | 9.30 (6.05,17.95) | -3.375 | 0.001 |
| Sodium, mmol/L, Median (IQR) | 136.00 (134.00,148.00) | 136.00 (134.00,138.00) | 135.00 (132.50,136.00) | -3.746 | <0.01 |
| IL-6,pg/ml, Median (IQR) | 76.77 (34.28,150.90) | 57.00 (26.65,124.50) | 174.00 (48.90,264.00) | -2.289 | 0.022 |
| procalcitonin, ug/L, Median (IQR) | 0.33 (0.15,1.10) | 0.32 (0.15,0.80) | 1.91 (0.29,5.48) | -5.169 | 0 |
Table 1: Baseline and clinical characteristics of KD patients in the two groups.
Twelve significant variables from univariate analysis, i.e., SAA, neutrophils, CRP, alanine aminotransferase, alanine aminotransferase, total bilirubin, lymphocytes, platelets, serum sodium, interleukin-6, procalcitonin and fever duration before initial IVIG treatment, showing a P value <0.05 were included in the multivariate analysis to identify risk factors for IVIG-resistance. Higher neutrophil levels, SAA and total bilirubin and lower platelet levels, serum sodium remained independent risk factors for IVIG-resistance in the multivariate analyses (Table 2).
| Variables | ß | Odds ratio (95%CI | P-value |
|---|---|---|---|
| Neutrophils, × 109/L | 0.123 | 1.131 (1.059-1.208) | 0 |
| Platelets, × 1012/L | -0.006 | 0.995 (0.991-0.998) | 0.004 |
| serum amyloid A, mg/L | 0.002 | 1.002 (1.000-1.003) | 0.008 |
| Sodium, mmol/L | -0.145 | 0.865 (0.767-0.976) | 0.019 |
| total bilirubin, µmol/L | 0.034 | 1.034 (1.013-1.055) | 0.001 |
Table 2: Multivariate logistic regression analyses of risk factors for IVIG-resistance.
ROCs were used to calculate sensitivity and specificity, and SAA exhibited a sensitivity of 69.8% and a specificity of 54.4% for predicting IVIG resistance at a cutoff of 252.45 mg/L. Regarding neutrophils, the cutoff was 9.88 × 109/L, and the sensitivity and specificity for predicting IVIG resistance were 72.1% and 59.0%, respectively. TBIL level had a sensitivity of 48.8% and a specificity of 79.5% for predicting IVIG resistance at a cutoff point 10.45 mg/L. A platelet cut-off value of 279.5 × 1012/L yielded a sensitivity of 51.2% and specificity of 73.2% for predicting IVIG resistance. At a cut-off of 136.5 mmol/L, sodium yielded a sensitivity of 81.4% and specificity of 46.4% for predicting IVIG resistance. The AUC of combined using these five indicators was 0.800, the sensitivity was 69.8% and the specificity was 76.2%. (Table 3, Figure 2).
| Variables | AUC | 95%CI | Cutoff Value | Sensitivity (%) | Specificity (%) | Youden index |
|---|---|---|---|---|---|---|
| Neutrophils, × 109/L | 0.683 | 0.598-0.769 | 9.88 | 72.1 | 59 | 0.311 |
| Platelets, × 1012/L | 0.643 | 0.555-0.730 | 279.5 | 51.2 | 73.2 | 0.244 |
| serum amyloid A, mg/L | 0.623 | 0.536-0.709 | 252.45 | 69.8 | 54.4 | 0.242 |
| Sodium, mmol/L | 0.674 | 0.590-0.757 | 136.5 | 81.4 | 46.4 | 0.278 |
| total bilirubin, µmol/L | 0.657 | 0.561-0.753 | 10.45 | 48.8 | 79.5 | 0.283 |
Table 3: Cut-off value of SAA for predicting IVIG resistance in KD patients.
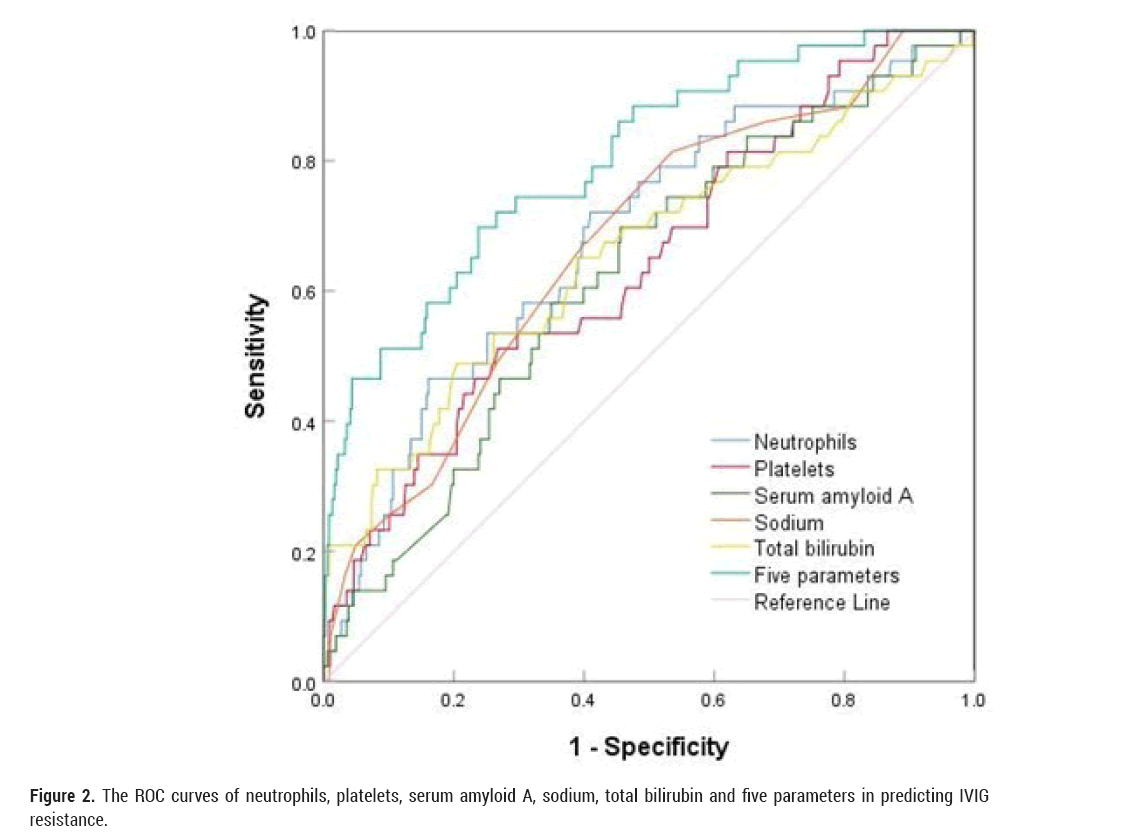
Figure 2: The ROC curves of neutrophils, platelets, serum amyloid A, sodium, total bilirubin and five parameters in predicting IVIG resistance.
IVIG resistance risk scores based on laboratory, echocardiographic, and/or clinical features have modest utility [17]. Nevertheless, several scoring models have been established to predict IVIG resistance in children with initial KD based on risk factors [6,24]. In general, specific, and reliable biomarkers for predicting treatment response in KD are urgently needed. To address this, we carried out this study to investigate the predictive value of SAA levels, alone and in combination with other parameters in KD. Predicting IVIG non-responders before initiating therapy can assist pediatricians in selecting more judicious and beneficial treatment. In this study, the IVIG resistance rate was 10.5%, consistent with previous reports [1].
The inflammatory responses are closely related to IVIG resistance. In our study, we confirmed significantly higher neutrophil levels in the IVIG resistant group, consistent with previous findings [6]. The clinical course of KD is closely related to platelet activation, and the Kobayashi risk score system established platelet count to predict IVIG-resistance [6,25]. Although the cause of hyponatremia is still unknown, there is a strong negative correlation between the serum sodium level and inflammatory factors, including CRP and IL-6. Hepatic dysfunction is a common complication during acute KD episodes and is characterized by elevated serum liver enzymes and hyperbilirubinemia. Hyperbilirubinemia is caused by lysis of hepatocytes and is indicative of intense inflammatory responses [12,26]. In our study, the higher level of total bilirubin in the IVIG resistant KD group was consistent with previous studies [24]. Severe immune-mediated inflammation of the blood vessels may be a cause of the related liver function injury [27].
The pathophysiology of the acute phase of KD is characterized by excessive activation of the innate immune system and is accompanied by an increase in inflammatory cytokines and chemokines [28,29]. Many classes of serum proteins are elevated in acute KD, such as CRP and SAA [22]. A truncated form of serum amyloid A has elevated in the plasma of KD [30]. SAA expression is typically induced in vivo during the inflammatory response and has been used as a biomarker for inflammation. Prior investigations into KD have largely focused on the association of other plasma inflammatory markers, such as CRP [31], which is a biomarker able to identify non-responders to IVIG. In the present study, we demonstrated that SAA levels were significantly high in the peripheral blood of KD during the acute phase. Our research found that higher SAA levels were independently associated with IVIG resistance, CRP levels were not significantly. It was found that the peak time of CRP and SAA level was not consistent, SAA may increase before CRP and fluctuate more sensitively in the acute phase of KD [22].
Five variables including SAA, neutrophils, sodium, platelets and TBIL were finally selected in the multivariate analysis. Neutrophils, sodium, platelets and TBIL have been identified as predictors in previous study. Regarding the predictive value of SAA levels, the sensitivity and specificity were 0.698 and 0.544, respectively, and the AUC was 0.623. In our study, SAA combined with neutrophils, sodium, platelets and TBIL showed higher efficacy for predicting IVIG resistance in KD, the AUC was 0.800. Both missed diagnoses and the misdiagnosis rate would be reduced. The mechanism is unclear that SAA is significantly increased in IVIG resistant KD patients. SAA-induced inflammation was markedly reduced by a neutralizing antibody against IL-17A in vivo [32]. So our results further strengthen to utilize SAA in assessing KD patients.
Study limitations
This study must be viewed considering some potential limitations. First, selective bias may have occurred, as this study was performed at a single institution. Second, the findings might be only applicable to KD patients receiving standardized IVIG treatment (2g/kg) prior to 10 days from fever onset. Last, because of non-routine testing, some reported risk factors for IVIG resistance were not considered in the study design, including levels of brain natriuretic peptide and immune function parameters. Also current prediction models are inadequate in distinguishing individual with high IVIG resistance in KD patients [33]. But when IVIG resistance is observed, second-line treatment with additional anti-inflammatory therapy is essential. Further studies are also necessary to understand the role of SAA more precisely in the pathophysiology of KD.
According to the findings of our study, The SAA level before IVIG was a complementary laboratory marker for IVIG resistance prediction in KD and may help identify high-risk patients for requiring additional clinical management.
This work was supported by the Key Laboratory Project of the Ministry of Birth Population Health (Grant number JK20213).
Huang XB participated in the design of the study, collected data, did the statistical analysis, and wrote the initial draft report. Zhao S, Liu ZY and Xu YY contributed to the data analysis and revised the report. Deng F contributed the design of the study and reviewed the manuscript, and approved the final manuscript as submitted. All authors have read and approved the submitted version.
Approval for this research was required from the Medical Committee of Anhui Provincial Children’s Hospital (Approval Number EYLL-2022-022), and an informed consent was obtained from the parents of each subject.
Citation: Deng F. Serum Amyloid A as a Biomarker of Immunoglobulin Resistance in Kawasaki Disease. ''J Biol Todays World, 2023,12(3), 001-006
Received: 15-May-2023, Manuscript No. JBTW-23-98776; Editor assigned: 17-May-2023, Pre QC No. JBTW-23-98776 (PQ); Reviewed: 01-Jun-2023, QC No. JBTW-23-98776; Revised: 09-Jun-2023, Manuscript No. JBTW-23-98776 (R); Published: 16-Jun-2023, DOI: 10.35248/2322-3308-23.12.3.002
Copyright: © 2023 Deng F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.