Review Article - (2023) Volume 12, Issue 4
The protective Angiotensin-Converting Enzyme 2 (ACE2) - essential part of the Renin-Angiotensin-Aldosterone System (RAAS) - is the target receptor for its physiological ligands Angiotensin I and II (Ang I/II) as well as for SARS-CoViruses and - spikes. It mediates Ang II degradation and is the precondition for SARS-CoVirus cell entry, spike-induced adverse reactions, and cytotoxic cell fusion. In spike-induced ACE2 downregulation, Ang II degradation is reduced with the consequence of increasing Ang II concentrations; the counter-regulatory protective Ang 1-7/AT2R/MAS axis is impaired.
The presented analysis provides a substantial body of evidence for the causal involvement of Ang II/activated RAAS in eliciting adverse reactions after application of spike-inducing vaccine. As an example, some serious organ disturbances or adverse reactions, in which the connection with an activated RAAS is obvious (cardiovascular and blood coagulation disorders, disorders of the nervous and muscular system, inflammatory reactions, auto-immunological, vascular and renal disorders), are presented and discussed.
Only a consistent refraining from any unfavorable influence on the highly complex RAAS enables harm avoidance. To limit or repair harm that has already occurred, RAAS inhibitors or recombinant ACE2 or ACE2 activators are suitable for spike-related symptoms. Based on individual assessment, the preference is currently for ARBs. An individualized approach depending on symptoms, individual conditions and differentiated diagnostics is essential.
Spike/SARS-CoV-2-interaction with ACE2 • Consequences of ACE2- down regulation • Cytotoxic cell fusion • RAAS activation • Spike/Ang II associated health disorders • Spike-induced adverse reactions • Treatment options
From the first observation of the numerous and unusual organ disturbances associated with a SARS-CoV-2 respiratory tract infection, the question of their specific cause arose. The virus-spike/ACE2/RAAS interaction and the resulting impairment of the organism's homeostatic regulatory systems and defence and repair mechanisms offered a plausible explanation. Since vaccination with spike-inducing vaccines, reports of adverse organ-related side effects have accumulated to an unusual extent, showing a broad spectrum of organ disorders analogous to COVID-19. However, the consequences of the proven interaction between viral/vaccine-induced spikes and their receptor enzyme, Angiotensin-Converting Enzyme 2 (ACE2-part of the Renin-Angiotensin-Aldosterone System (RAAS)), in triggering adverse reactions have been largely ignored, despite signalling a causal relationship.
This analysis evaluates the scientific evidence for the hypothesis of the fundamental role of ACE2 downregulation/RAAS activation and the influence of its essential components in triggering numerous spike-induced vaccine side effects. Possible consequences for diagnostic and therapeutic intervention options are extrapolated. Local reactions, allergic/hypersensitivity reactions and reactions to other components of the finished vaccine are not the subject of this analysis.
The key function of Angiotensin Converting Enzyme 2 (ACE2)
The enzyme ACE2 plays a key role in understanding organ impairment caused by SARS-CoV-2 infection and systemic complications of spike-inducing vaccines. Its physiological role is that of a counterregulator in an activated Renin-Angiotensin-Aldosterone System (RAAS) with principally cardio- and tissue-protective effects. ACE2 is also considered an indispensable mediator of spike-induced cell fusion. Pathogenic SARS-CoViruses and non-neutralised vaccine spikes use this enzyme as a target receptor in competition with the natural ligands angiotensin I/II, whereby the protective function of this enzyme is lost and the gateway opens for disturbances of homeostatic regulatory systems and of defence and repair mechanisms, such as hyperinflammation, remodelling, thrombo-embolic or immunological disturbances.
The Angiotensin-Converting Enzyme 2 (ACE2) protein is a transmembrane protein with an extracellular catalytic substrate-binding domain, a transmembrane region and an intracellular residue [1]. A second form is the soluble one, which circulates in the body fluids. Its natural ligands-Angiotensin I (Ang I) and the much more significant, pathophysiologically potentially harmful Angiotensin II (Ang II) are hydrolysed to Angiotensin 1-9 (Ang 1-9) and the protective heptapeptide Angiotensin 1-7 (Ang 1-7), respectively. Ang 1-7 can also be produced directly from Ang II by a Propylcarboxypeptidase (PRCP), by the Prolyl Oligopeptidase (POP) or by a Neutral Endopeptidase (NEP) [2]. Ang 1-9 can also be further cleaved to Ang 1-7 under the influence of neutral peptidases or ACE. These angiotensinases are active in a tissue-specific manner (POP in the bloodstream and lungs; PRCP ubiquitously, affecting cell proliferation, autophagy, oxidative stress, inflammatory responses, vascular homeostasis/endothelial dysfunction) and are able to attenuate the deleterious consequences of ACE2 functional impairment [2]. Increased POP/PRCP activities were found in older age and comorbidity (obesity, cardiovascular risk factors, atherosclerosis/unstable plaques, renal disease, inflammation, diabetes), but not in younger age groups. This may be why Ang II-induced vaccine side effects are more common in younger people or healthy individuals [2]. In addition, ACE2 expression is reduced in children, increasing the consequences of Ang II accumulation [2]. On the other hand, this finding provides a possible explanation for the lower frequency and severity of COVID-19 disease in children [3].
The affinity of ACE2 for Ang II is significantly higher than for Ang I; the catalytic efficiency for Ang II is approximately 300-fold that for Ang I [1].
Auto-antibodies against ACE2 may impair the functionality of this protective enzyme. In COVID-19 sufferers, auto-antibodies against ACE2 increased and a positive correlation was found between them and antibodies against S1-RBD; with significantly lower binding affinity to ACE2, antibodies reacted with both S1-RBD and ACE2 possibly based on antigen similarity. The antibody-response may thus exert a dual role-protection and immunopathogenesis [4]. It is discussed whether the increasing soluble plasma ACE2 as antigen under certain circumstances could trigger auto-immune processes against membrane-bound ACE2 [5].
Importance of ACE2 for spike efficacy: The high-affinity interaction between SARS-CoV spikes (structural protein subunit S1 with open receptor-binding domain-RBD) and the ectodomain of the receptor enzyme ACE2 is scientifically well established; it is of fundamental importance for virus entry into host cells and for mediating the effect of non-neutralised vaccine spikes see Figure 1. The open form of the S1-RBD, present in only about 20%, increases the probability of recognition by host antibodies [4]. The spike RBD performs a dual function: with its three non-overlapping antigenic sites, it is the primary target of competing antibodies [6]. Concomitantly, spike binding triggers functional impairment of ACE2 with pathophysiologically relevant consequences.
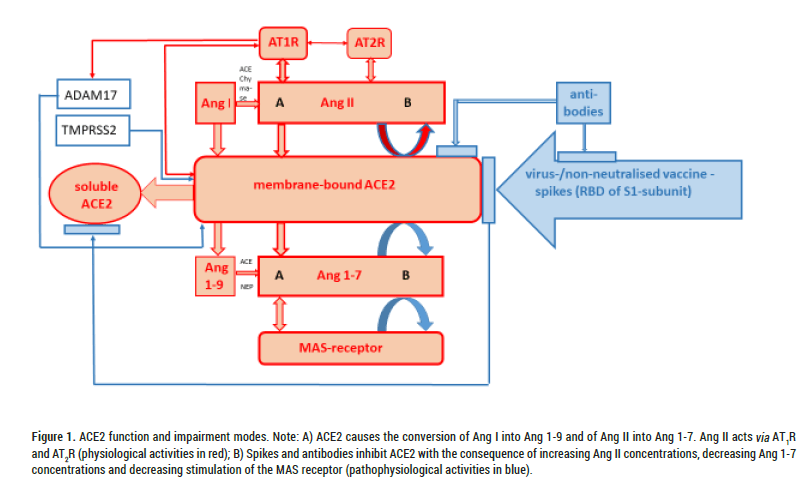
Figure 1: ACE2 function and impairment modes. Note: A) ACE2 causes the conversion of Ang I into Ang 1-9 and of Ang II into Ang 1-7. Ang II acts via AT1R and AT2R (physiological activities in red); B) Spikes and antibodies inhibit ACE2 with the consequence of increasing Ang II concentrations, decreasing Ang 1-7 concentrations and decreasing stimulation of the MAS receptor (pathophysiological activities in blue).
How does the interaction between the SARS-Co virus/spikes and ACE2 work?: According to current knowledge, it can be assumed that all SARS-CoV-2 variants and "escape mutants" bind to their receptor enzyme ACE2 with the S1 subunit of the S protein [7].
Virus-cell membrane fusion is influenced by various factors (e.g. pH, cleavage of the S protein, activation of the fusion proteins, temperature). It has been proven that the lack of ACE2 receptors prevents fusion with the S protein [8].
The first step of virus fusion with host cells is the furin-dependent cleavage of the SARS-CoV-spike-glycoprotein into two subunits-S1 (containing the receptor-binding domain RBD) and S2/S2 (membrane fusion effector). The subsequent possible complete fusion of the viral envelope proteins with those of the host cell membrane can only take place after a further cleavage (S2`). This requires a sheddase, the pH-independent cell surface Transmembrane Serine Protease2 (TMPRSS2) (fast entry pathway), which influences the cytoplasmic domain of ACE2. Increased TMPRSS2 activity associated with virus entry triggers reduced membrane-bound ACE2 activity with the consequence of an increase in Ang II and AT1-Receptor (AT1R) activity and reduced conversion to the protective Ang 1-7 as well as reduced MAS receptor activity see Figures 1 and 2. SARS-CoV-2 infection can also block the catalytic activity of soluble ACE2 and thus additionally prevent the metabolisation of Ang II into Ang 1-7 in plasma.
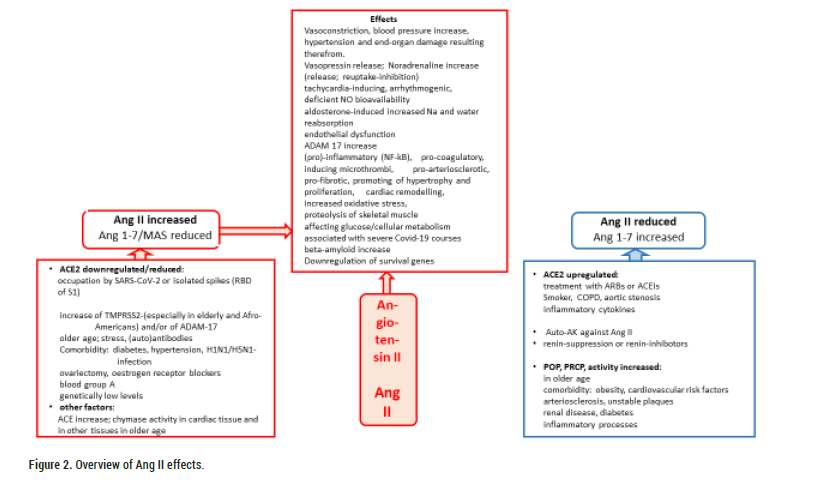
Figure 2: Overview of Ang II effects.
The Ang II/AT1R activity increase simultaneously causes increased ADAM17 activity. ADAM17 is another sheddase that acts on membrane-bound ACE2 and induces its conversion to a soluble form that is still biologically active. It has been shown that Ang II stimulated the conversion of membrane-bound ACE2 into the soluble ACE2 form present in plasma (ACE2 shedding with the help of TNF alpha-converting enzyme TACE=ADAM17) and consequently the membrane-bound form was reduced. This process could be prevented by Angiotensin Receptor Blockers (ARBs) [9].
The loss of myocardial ACE2 and the increase in soluble ACE2 is similar to the increase in soluble ACE2 activity in plasma observed in patients with heart failure. In advanced, significant cardiac dysfunction, the plasma ACE2 increase correlates with the degree of deterioration of cardiac function. The activation of pro-inflammatory substances by Ang II can trigger cardiovascular complications in inflammatory diseases in connection with ACE2-shedding [9].
However, in the absence of TMPRSS2 or its insufficient presence in target cells, viruses can also enter endolysomes by endocytosis, from where they are activated in a pH-dependent manner to reach the cytosol at a later time [6,10,11]. The endolysosomal entry mechanism is apparently less efficient for SARS-CoV-2, which could explain the low infection-inhibiting efficacy of hydroxychloroquine [6].
Elevated TMPRSS2 levels have been found in the elderly and African-Americans, which could be a possible explanatory factor for the increased severity of COVID-19 disease in these populations [12].
It has been known since 2005/2006 that the SARS-CoV spike protein is capable of down-regulating ACE2 in vitro and in vivo in the absence of other viral components, leading to conditions resembling those of ace2-knock-out mice [13,14]. The Ang II increase in spike-treated mice was attenuated by AT1R blockade.
In 2020, it was demonstrated that spike protein (S1 region) expression alone in human lung epithelial cells was effective in inhibiting ACE2 expression, inducing increased Ang II levels and initiating the signalling cascade mediated after significantly increased AT1R expression, including ADAM17 induction and inflammatory markers (IL-6 and other cytokines) [15].
Lei, et al. later described damage to vascular endothelial cells in animal experiments by ACE2 downregulation, impaired NO bioavailability and inhibition of mitochondrial functions in response to spike proteins-preconditions for development of endotheliitis. Endothelial cells with reduced ACE2 stability were found to have more fragmented mitochondria with reduced basal respiration, reduced ATP production and maximal respiration than ACE2-overexpressed endothelial cells, but had increased basal acidification rate, glucose-induced glycolysis, maximal glycolytic capacity and glycolytic reserve [16]. S1 protein treated endothelial cells accordingly showed decreased mitochondrial function but increased glycolysis. The S protein may lead to ACE2 destabilisation via increased redox stress and deactivation of AMP kinases.
The risk of S1-induced endothelial damage and other spike-associated disorders must be weighed against the potential benefit of reduced viral infectivity through ACE2 downregulation.
Another deleterious attribute of SARS-CoV-2 spike proteins is their ability to fuse cells [17,18]. In addition to alveolar destruction, thrombosis and fibrotic changes, abnormal, syncytially altered pneumocytes with multiple nuclei (2 to >20) were found in approximately 90% of cases in those who died of COVID-19 [18]. Experimentally, it was already proven some time ago by use of a cell-cell fusion assay that cells expressing SARS CoV-S proteins on their surface can fuse with adjacent cells and form syncytia if these neighboring cells are equipped with the receptor ACE2 [14,19]. Pathological cell fusion thus requires the existence of ACE2 and is likely to affect its function.
Recently, the SARS-CoV-2 spike protein has been described as "enormously fusion active" [20]. Minimal amounts of spike protein on the cell surface or spiked virus particles are sufficient to initiate fusion, even if ACE2 is not overexpressed. Interestingly, neutralising antibodies prevented membrane fusion of virus particles with cells highly efficiently, but fusion of spike-bearing cells with each other much less so, so that a different mode of fusion between virus-infected cells with neighbouring cells and cell-cell fusion by means of external particles ("fusion from outside") is assumed [21]. Alteration of the spike structure can significantly increase or change the fusion activities without exerting much influence on the virus/cell fusion [17]. On the basis of these findings, it can be assumed that the spikes produced after vaccination can lead to pathogenic cell fusion and produce similar undesirable effects as with COVID-19 [17]. Looking for options to inhibit cell fusion or to provide protection against virus-induced cytopathic effects, Braga, et al. identified several potential candidates (e.g. niclosamide) among 3825 screened drugs that had in common the inhibition of SARS-CoV-2 spike-induced changes in intracellular calcium oscillations involving the scramblase TMEM16F [18]. Downregulation of TMEM16F decreased syncytia formation in spike-expressing cells, similar to anti-ACE2 siRNA; overexpression of TMEM16F significantly stimulated SARS-CoV-2 spike-induced syncytia. Consequently, an increase in activity of TMEM16F is hypothesised as a common mechanism of spike-dependent cell-cell fusion; furthermore, an involvement of TMEM16F in inflammatory and thrombotic processes as well as in the development of endothelial dysfunction, alveolar oedema and diarrhoea is discussed.
Cell-cell fusion has also been identified as part of the efficient cell-cell transmission/infection triggered by the SARS-CoV-2 spike protein [22]. This could explain the unusual frequency of pathological pulmonary symptoms after mRNA-vaccine application.
The uniqueness of the spike-inducing mRNA-vaccines is that, in contrast to conventional vaccines, not a well-dosed and sufficiently tested antigen is applied, but only the mRNA with the genetic code for production of one of several antigens of the SARS-CoV-2 virus. Moderna, Biontech and Novavax introduced some proline mutations into the spike protein with the aim of thereby keeping the spike protein produced in its prefusion conformation for longer [23]. Neither a more sustained antibody-production nor a reduced side effect rate is proven. It is rather likely that the consequences of an undesirably prolonged ACE2 functional impairment will increase. Related results are not available.
Once the mRNA with the genetic code for antigen production arrives in the host cytosol, ribosomal translation begins; the specific spike glyco-protein (subunit S1 with Receptor-Binding Domain (RBD)) is generated. This intracellularly constructed antigen is presented on the cell surface after completion and is available for the desired antibody production and the triggering of further reactions (cell fusion, ACE2 interaction). In contrast to the known spike equipment of SARS-CoV viruses, the amount, duration of formation, its influenceability and the systemic availability of spikes after application of spike-inducing vaccines have not been systematically investigated so far, although their knowledge would be extremely important for estimating vaccination success and tolerability. For the first time, it was recently found by two different methods that about 1 pmol of spike protein is formed per 6 pmol of mRNA vaccine [24]. Further details and comparisons are lacking. The studies on the distribution of lipid nanoparticle-coated luciferase mRNA is only indirectly suitable for characterising spike production and distribution [25]. They only prove that detectable or highest concentrations were already present at the first time of examination, 15 minutes after application (in the case of radioactively labelled LNP luciferase mRNA) or 6 hours after application (lipid nanoparticle-coated luciferase mRNA) [25]. An onset of action is therefore not to be expected before these vaccine concentrations are reached. Doubts about the systemic distribution of the vaccine and the associated spike presence were disproved. Spikes are systemically present in the human organism and detectable for longer than expected with corresponding consequences-rapid, haemodynamically effective as well as delayed reactions over days to weeks.
Separating the immune response from the undesirable and deleterious consequences of ACE2 downregulation or RAAS activation seems impossible because the same Receptor Binding Domain (RBD) of the interacting spike S1 subunit is engaged for both [26].
In contrast to conventional vaccines, the novel spike-based vaccines are characterised by their comparatively unusually extensive, class-specific spectrum of side effects [27,28].
Consequences of ACE2 down regulation: The manifestation and severity of COVID-19 disease obtain a completely new dimension among viral respiratory diseases due to the downregulation of the SARS-CoV receptor ACE2, which applies in the same way to the a priori foreseeable consequences of an interaction between non-neutralised vaccine spikes and ACE2.
ACE2 is expressed in almost all vital organs and organ systems, but to varying degrees organ-specifically, and thus accounts for the organ tropism of SARS-CoV-2, which extends far beyond the respiratory tract. In addition to alveolar epithelial cells, ACE2 is particularly highly expressed in pericytes, which are highly concentrated in cardiac muscle tissue. ACE2 is also highly expressed in the intestine, testes, kidney and thyroid [6,29-31]. ACE2 protects the lung from severe acute damage caused by SARS by negatively regulating Ang II, as was proven experimentally some time ago [14].
Of essential importance for SARS-CoV-2 and spike-based vaccine-related organ injuries is that ACE2 is an integral component of the extremely important and highly complex Renin-Angiotensin-Aldosterone Regulatory System (RAAS). ACE2 is responsible for Ang I/II degradation and is thus an important modulator in limiting the harmful consequences of Ang II activation. Detailed experimental findings on the mode of action and counter-regulatory influencing of various components of the RAAS, in particular the local and intracellular ones, but also clinical findings have exploded in recent years and in some cases provide new perspectives on the physiology and pathophysiology of this regulatory system [32].
Convincing evidence for the crucial role of ACE2 is provided by the consequences of downregulation or low expression of ACE2 (e.g. through viral occupation, receptor occupation with non-neutralised vaccine spikes, by diabetes, hypertension, stress, H1N1 and H5N1 infection, in old age, ovariectomy or in connection with oestrogen receptor blockers, in people with blood group A or/and genetically determined low levels). The resulting activated RAAS triggers a cascade of systemic consequences up to the so-called "Ang II storm or intoxication “with hyperinflammatory conditions.
Individuals with low baseline ACE2 levels are at particular risk of adverse effects if viral or vaccine spikes additionally downregulate ACE2 [2,33]. The therapeutic use of a recombinant human ACE2 therefore appears promising [7].
The principally protective and anti-pathogenic ACE2/Ang 1-7/MAS axis as well as AT2-receptor effects are increasingly impaired in conditions of ACE2 loss of function; vasodilatory, antihypertensive, antiproliferative, myocardial-protective, antiarrhythmic, positively inotropic and anti-inflammatory as well as insulin resistance-reducing Ang 1-7 effects are diminished [1]. The combined loss of ACE2 and MAS-activity significantly increased the severity of complications [34].
The observed increase in myocardial ACE2 concentration and activity during treatment of heart failure patients with Angiotensin Converting Enzyme Inhibitors (ACEI) and ARBs requires further clarification and interpretation [9]. Animal experiments showed predominantly ACE2 expression after ARB treatment; the findings after ACEI treatment were not consistent. In hypertensives and in patients with diabetic nephropathy, increased renal ACE2 excretion was diagnosed after chronic ARB treatment, the relevance of which is under discussion [35,36].
Inflammatory cytokines (IL-1beta, type 1 and III interferons) increased ACE2 expression in critically ill COVID-19 patients [6]. Up-regulation of ACE2 has been observed in aortic stenosis at older ages (soluble ACE2), in smokers and in patients with chronic obstructive pulmonary disease, but is controversial discussed [3,6,37].
Angiotensin II
The multifaceted, multipotent octapeptide Angiotensin II (Ang II) is the unquestioned major effector in the RAAS and is implicated in the primary response to SARS-CoV-2 infection as well as in the response to non-neutralised vaccine spikes. Ang II affects the function of almost all organs, including the heart, kidney, vascular system and brain [38]. Therefore, knowledge of Ang II effects is an indispensable precondition for understanding adverse reactions associated with spike-inducing vaccination and possible therapeutic approaches.
Ang II is primarily produced in the vascular endothelium and can also be synthesised locally, e.g. in the brain, independently of its systemic availability [39,40]. Renin catalyses the conversion of angiotensinogen into the biologically weak Ang I, which is converted by the Angiotensin-Converting Enzyme (ACE) into Ang II and subsequently into Ang III and IV. High Ang II concentrations are limited by negative feedback via AT1R and with the involvement of AT2R by suppressing renin biosynthesis [41].
Ang I and II are, as already mentioned, the physiological ligands of the enzyme ACE2, which hydrolyses them into Ang 1-9 and Ang 1-7, respectively. Numerous experimental and clinical findings demonstrate an inverse relationship between ACE2 activity and Ang II concentrations.
Ang II concentrations are controlled in principle by the balance between ACE and ACE2 activity, alternatively to angiotensin-converting enzyme in cardiac, vascular and renal tissues by chymase produced by mast cells [40]. Fatal is chymase activity in cardiac tissue of older age with markedly elevated Ang II concentrations that do not respond to ACE inhibition [31]. In contrast, chymase inhibitors showed cardiac tissue-protective properties in experimental studies.
The discovery of local, paracrine and autocrine, tissue-specific RAA systems (e.g. renal parenchyma, immune system) provides explanations for local modulation of the efficacy of the circulating RAAS and, independently, for tissue-specific effects of a non-haemodynamic nature.
Transmission of Ang II effects: Ang II effects are mediated by both AT1-and AT2-receptors see Figures 1 and 3, with AT1R implicated in classically physiological but also pathophysiologically deleterious, and AT2R in mediating vasodilation and further protective effects [39,41,42].
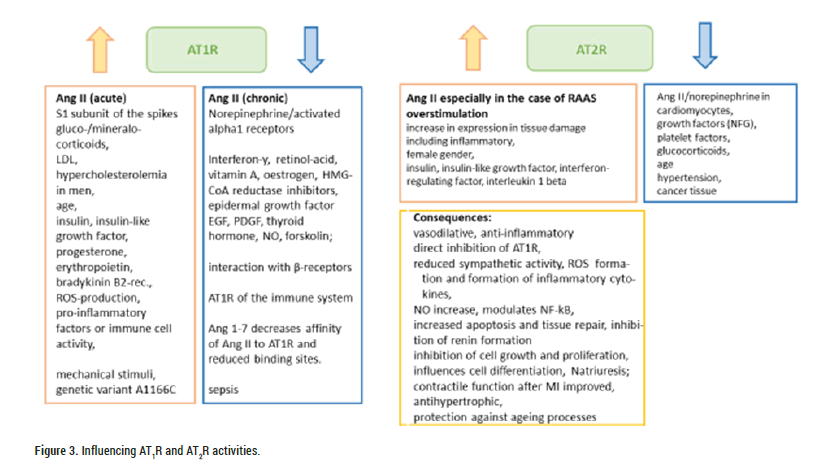
Figure 3: Influencing AT1R and AT2R activities.
High AT1R expression is particularly present in vascular smooth muscles. Of importance is the presence of AT1R in macrophages, bronchial epithelium, zona glomerulosa of the adrenal glands, endometrium and dopaminergic brain regions.
In adults, AT1R is more highly expressed than AT2R in cells of the immune system (macrophages and T-cells). In preclinical studies, inhibition of differentiation of these cells and reduction of proinflammatory consequences were observed after AT1R activation. Normally, these cells produce hypertensive kidney damage without inhibition. Therefore, the AT1-receptors of the immune system are thought to have a protective effect on pathogenic renal and vascular AT1R actions in hypertension to limit tissue damage [43].
AT1R is also likely involved in tumour angiogenesis, as an AT1R antagonist has been shown to prevent tumour growth and angiogenesis [44].
Acutely elevated Ang II concentrations increase AT1R activation; however, chronically elevated Ang II concentrations downregulate the activity of the receptor AT1, which is subject to multiple other influences (stimulatory/upregulatory: gluco-/mineralocorticoids, LDL, hypercholesterolaemia in Men, insulin, insulin-like growth factor, progesterone, erythropoietin, bradykinin B2 receptor; inhibitory/down-regulatory: Interferon-y, retinol secretion, Vitamin A, oestrogen, HMG-CoA reductase inhibitor, epidermal growth factor EGF, PDGF, thyroid hormone, NO, forskolin; interaction with β-receptors; various pathophysiological states, such as sepsis) [38,39]. In the same direction points the finding that renal AT1R inversely correlates with RAAS activity. Ang 1-7 decreased the affinity of Ang II for AT1R and reduced binding sites [39]. Systemic AT1R downregulation with non-response to Ang II in sepsis could have fatal consequences for severe SARS-CoV2 infections.
Ang II signal transduction: Ang II signal transduction starts with AT1-receptor-dependent G-protein activation modulated by tyrosine kinases and results in intracellular calcium release or increase, which induces vascular smooth muscle contraction. The increase in Reactive Oxygen Species (ROS) due to induction of the NADPH oxidases Nox 2 and 4 also plays a crucial role in pathophysiological Ang II effects [45,46]. Numerous redox-dependent processes are controlled by subtle regulation of ROS and by antioxidant enzymes. Noxs are activated by growth factors, such as PDGFR, EGFR and cholinergic receptors, and their expression is increased by Ang II. Interestingly, Nox-generated ROS can stimulate other ROS-producing systems and thus multiply the extent of oxidative stress in associated diseases [45].
Noxs are expressed in cardiomyocytes and all cells of the vessel wall (endothelium, smooth muscle cells, and fibroblasts of the adventitia). They regulate cell differentiation, proliferation, migration, angiogenesis and vascular tone. Under the influence of Ang II, vascular Nox (Nox 1, 2, 4 and 5) increase blood pressure by enhancing adrenergic vasoconstriction and direct effects on vascular smooth muscle [45]. In addition, it was demonstrated years ago that Nox-dependent ROS production activates the AT1-receptor through a self-amplifying effect and potentiates cell signalling [45]. Nox2 elevation or Nox dysfunction correlates with hypertension. The Nox-mediated increase in superoxide concentration after Ang II can be prevented by AT1R-antagonists, especially in patients with coronary artery disease [45].
Other pathophysiological factors involved in Ang II-induced organ injuries are loss or reduction of NO bioavailability, increased oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, interference with transcription factors, release of inflammatory cytokines (IL-6, TNF-α) by endothelial cells and/or other cells, expression of adhesion molecules, activation of NF-ĸB, Toll Like Receptor 4 (TLR4) crosstalk, increased release of microparticles/exosomes and receptor-mediated transduction of mechanical stimuli [32]. Mechanical or biochemical stimuli are at the beginning of pathophysiological sequelae; they are considered to trigger the release of vasodilatory (nitric oxide NO, prostacyclin PGI2) or vasoconstrictive mediators (endothelin) by endothelial cells [47]. NF-ĸB activation plays a key role as a link between increased Ang II levels, e.g. in SARS-CoV-2 infection, and inflammatory cell attraction, increased cytokines/chemokines and PDGF-B expression [48].
AT1R is subject to a genetic polymorphism that has been associated with increased development of cardiovascular risk factors, such as increased sensitivity to vasoconstrictor Ang II effects. The genetic variation A1166C has been observed in hypertension, myocardial infarction/coronary heart disease, hyperlipidaemia or aortic vascular disease [38].
Angiotensin II-mediated vasoconstriction: Vasoconstrictive, blood pressure-increasing efficacy is the most well-known physiological effect of Ang II, with pathophysiological deleterious consequences in the presence of activated RAAS, comorbidity and sustained efficacy. Ang II efficacy is realised by involving central AT1-receptor-rich/Ang II-producing regions and influencing central sympathetic catecholaminergic activity with the aim of maintaining plasma volume homeostasis. A rapid pressure response occurs via changes in peripheral vascular resistance, while a protracted pressure response is based on increased sodium reabsorption involving aldosterone in the kidney and increased sympathetic tone. Aldosterone, in addition to its plasma volume expanding effects, is also involved in vascular inflammation, oxidative stress, fibrotic activity, remodelling and endothelial dysfunction [31]. In the periphery, Ang II causes increased norepinephrine release while inhibiting its reuptake at nerve endings, thereby increasing vasoconstriction and increasing heart rate and cardiac output.
Ang II and inflammatory processes: A large number of findings suggest that the RAAS/ACE2/Ang II system is fundamentally involved in inflammatory processes and in disorders of both the innate and acquired immune systems [3].
The RAAS signaling cascade interacts with the immune system on several levels, both systemically and tissue-specifically. Thus, in addition to catalytic properties, renin also possesses pro-inflammatory properties mediated via its receptor, which can lead to inflammatory reactions, e.g. in vascular tissue or microglia. In contrast, unimpaired ACE2 function, presumably by enhancing Ang II degradation and increasing Ang 1-7 concentration, ameliorates inflammatory lesions in the kidney and vasculature and limits the production of pro-inflammatory cytokines (TNF-alpha, IL-6) by macrophages. An interesting finding in recent years is that the AT1-receptor is more strongly expressed than AT2R in cells of the immune system in adults. It is thought that the AT1-receptors of the immune system modulate pathogenic renal and vascular AT1R effects to limit tissue injuries in hypertension. Independently, the AT2-receptor is considered to counteract AT1R effects and thus possesses a broad spectrum of anti-inflammatory properties. The development of Ang 1-7 analogues and specific AT2R-agonists is thought to have therapeutic potential [43].
The first evidence for the involvement of the RAAS in inflammatory responses dates back to 1978, when it was discovered that Ang II has specific binding sites on human leukocytes and that the Ang II-generating enzyme ACE is more strongly expressed in granuloma tissue [49,50]. It has been known for decades that immune cells (e.g. macrophages in granulomas or circulating leukocytes) can generate and release Ang II, that AT1 receptors are present in T cells, macrophages and in lymphoid organs and that Ang II can therefore act directly on immune cells and exert proliferative effects [51-53].
AT1R inhibition with anti-inflammatory and immunosuppressive efficacy, e.g. in immunologically caused myocarditis, to suppress pathogenic Ang II effects, was already considered therapeutically useful at that time. The favourable effects on the vascular musculature and the inhibition of the production of inflammatory mediators have been attributed a significant role [53].
Infiltrating leukocytes are able to generate components of the RAAS in the target tissue, initiating further destruction. In kidney, heart and vessels, Ang II triggered an inflammatory response by promoting the expression of pro-inflammatory cytokines/chemokines responsible for the accumulation of immunocompetent cells in the tissue. Vascular Endothelial Growth Factor (VEGF) and adhesion factor expression were increased. Cox-2 activation has been implicated in mediating endothelial dysfunction, activation of dendritic cells in pro-inflammatory activities. TLR4 expression is mediated via activation of AT1R. In contrast, AT1R blockade or ACE inhibition reduced inflammatory processes and protected tissues from oxidative lesions independently of blood pressure reduction [40,43].
Local Ang II formation can modulate inflammatory responses by altering blood flow, vascular permeability and granulomatous reactions [52].
Of considerable clinical relevance is that cytokines released by activated endothelial cells can result in microcirculatory lesions with pro-coagulatory consequences [31].
SARS-CoV-2 infection causes an imbalance in the RAAS resulting in increasing levels of pro-inflammatory molecules and inflammatory responses up to cytokine storm. The presence of SARS-CoV-2 spike protein in epithelial cells promoted IL-6 trans-signalling through activation of the AT1-axis, aiming to initiate a hyperinflammatory response [15].
Stressed COVID-19 sufferers or those vaccinated with spike-based vaccines are exposed to dual RAAS activation, namely stress-induced by increased sympathetic tone/Ang II concentrations and spike-induced activation of the RAAS with increasing Ang II concentrations, which may not only trigger acute haemodynamic consequences, but may furthermore result in an inflammatory process or thrombo-embolic consequences. Post-mortem reports from COVID-19 patients indeed demonstrated severe vascular damage and alveolar microthrombi [31].
Further Ang II effects: Ang II controls cellular growth (hypertrophy and proliferation), adhesion, migration, intracellular matrix deposition and thereby influences chronic adaptive processes in blood vessels and the heart muscle, such as so-called remodelling, as well as tissue repair and development of arteriosclerosis [39]. Experimentally, the influence of AT1R activation on the electrical conductivity of myocytes proved to be relevant with regard to the triggering of a ventricular arrhythmia [39]. Other effects, such as pro-fibrotic, affecting glucose metabolism and cell ageing or leading to proteolysis of skeletal muscle, complete the pathophysiological spectrum of increased Ang II activity.
In 2017, it was discovered that significant expression of AT1R and MAS is present in bladder cancer tissue, confirming previous findings [44].
ACE2/Ang1-7/MAS axis and AT2R: The aforementioned anti-pathogenic ACE2/Ang 1-7/MAS axis and AT2-receptor activation are protective and anti-inflammatory. AT2R expression is limited in adults, but it increases in response to tissue damage and was found to be particularly highly expressed in females [31,41]. In cells of the immune system, AT2R is not particularly highly expressed [43]. Activation of AT2R (see Figure 3) begins with Ang II binding (in certain tissues, Ang III is the preferred agonist). It is considered beyond doubt that the vasoconstrictor Ang II/AT1R effects are antagonised and AT2R-mediated vasodilation in resistance and capacitance vessels or hypotension prevails, which is not only acutely detectable but also longer lasting; desensitisation is not known [41]. In particular, when AT1R/activated RAAS is overstimulated (e.g. during salt restriction or Ang II infusion), the protective vasodilatory effect of AT2R comes into play; an inverse relationship exists between AT1R and AT2R (see Figure 1). Independent of agonist activation, AT2R can directly trigger inhibition of AT1R [38,39,54].
Activation of the AT2-receptor reduces ROS formation and inflammatory cytokine activity, increases NO formation, modulates NF-ĸB, increases apoptosis and tissue repair mechanisms (including neuronal), inhibits renin formation, cell growth and proliferation, influences cell differentiation, reduces sympathetic activity, induces natriuresis and participates in blood pressure regulation, can improve contractile function after myocardial infarction and thus counteracts the development of cardiac hypertrophy. In addition, some protection against ageing processes and related chronic diseases is conceivable.
Under pathological conditions, such as tissue and vascular damage, myocardial infarction, heart failure, renal failure or brain ischaemia/peripheral nerve damage, an activation of AT2R expression has been described. In 2017, it was found for the first time that AT2R is markedly expressed in the inflamed synovial tissue and in cells of the innate and adaptive immune system of patients with rheumatoid arthritis, less markedly in osteoarthritis. In this context, it is discussed that the overexpression represents an anti-inflammatory containment mechanism of the cellular response to Ang II [55].
Ang II and noradrenaline caused downregulation of AT2R in cardiomyocytes, growth factors and glucocorticoids in several other cell types [39]. In the vascular smooth muscle of hypertensives, a marked decrease in AT2R expression was found compared to normotensives and it was therefore concluded that restriction of the AT2R signal transduction pathway contributes to hypertension development [56].
In bladder cancer tissue, AT2R was significantly downregulated; enhanced induced overexpression triggered apoptosis involving further factors (BCLA1, TNFSF10, DRs) and suppressed cancer cell proliferation [44].
Ang 1-7 is thought to have vasodilatory and anti-thrombotic effects (through NO activation), as well as anti-fibrotic and anti-inflammatory effects [31]. Its effects are mediated via the Mas receptor, which, like the AT2R, produces tissue-protective and regenerative effects [44]. It attenuates fibrosis, proliferation and remodeling processes; pro-inflammatory effects are partially prevented [3]. MAS loss resulted in impaired cardiac function and increased fibrosis [32]. Loss of ACE2 has been associated with cardiac hypertrophy and fibrosis; cardiac remodelling and its consequences, including fatal outcome, and rising Ang II levels have been observed in diabetics or after myocardial infarction. ACE2 and MAS complement each other in their protective efficacy against Ang II-mediated outcomes [34].
Recent studies have identified two other angiotensins, Ang III and IV. Ang IV/AT4R appears to prevent proinflammatory effects, exerts cardioprotection and cerebrovascular protective effects in inflammatory diseases and has anti-apoptotic effects [3].
Selected spike/angiotensin II-associated health disorders
The consequences of increased Ang II activity or dysregulated RAAS are extensive and well established. Their relevance in causing numerous extrapulmonary organ injuries in COVID-19 disease is recognised. Ang II plays a crucial role in the development of severe courses of COVID-19; a direct relationship could be established between high Ang II levels/ACE2 deficiency and COVID-19 severity [2,3,48,57]. On the other hand, antibodies against Ang II were found in a significant proportion of hospitalised patients (63%), but these were not consistently detectable during the course of the study [58].
This causality has not yet been considered with regard to side effects of the spike-inducing vaccines, although they are based on the same mode of action-spike-induced downregulation of ACE2 and activation of the RAAS. As an example, some health disorders that can be assumed with a high probability to be related to spike-based induction are addressed below.
Cardiovascular disturbances: The most significant effect of Ang II is undoubtedly the hypertensive effect, which can manifest itself acutely as hypertensive crisis, tachycardia/arrhythmia, acute left heart failure, ischaemia-related cardiac or CNS-symptoms, myocardial infarction, sudden (cardiac) death or stroke (ischaemic or haemorrhagic). An analysis of suspected cardiovascular side effects of spike-based vaccines reported to the European Medicines Agency (EMA) showed a dramatic accumulation (3-4 digit ranges) of these, sometimes life-threatening, class-specific, spike-associated side effects already within the first 5 months since the start of the vaccination campaign. Blood coagulation disorders, embolisms, cases of thrombosis, myocarditis and vasculitis completed the spectrum [27]. The importance of acute cardiovascular reactions is underlined by the fact that deaths caused by them accounted for at least one third of all deaths associated with this vaccination. Despite the high plausibility of the presented causal relationships between spike-induced activated RAAS and acute cardiovascular disorders, only myo-/pericarditis and thrombosis were attributed a vaccine-related signalling effect by the EMA with reference to other trigger mechanisms. Neither the special distribution of ACE2 in the pericytes of the capillaries and small blood vessels of the myocardial tissue, in cardiomyocytes and endothelia, nor a pre-existing low ACE2 level linked to high Ang II concentrations, as in the multiple stressed older age (increased chymase and TMPRSS2 activity; see Figure 2), was discussed as an explanation for the pathogenesis of the globally declared "myocarditis" [29]. Direct spike damage potential [16], genetically fixed low ACE2 activity or a situation additionally aggravated by stress were also not considered. Specific investigations failed to materialise. Even an alarming signal-the sudden cardiac death of two adolescents- was not used to clarify further "myocarditis" cases, despite resolute references by the authors to the suspected underlying stress cardiomyopathy caused by catecholamines (one could also say: toxic Ang II/NA storm) [59]. In Germany, officially reported deaths did not decrease at all from the start of the vaccination campaign on 27./28.12.2020 to 18.3.2021, but increased to 44006 deaths within the first 81 days compared to the previous value of 30126 deaths without vaccination (until 28.12.2020). Compared to reported vaccination deaths of previous years, those after spike-based vaccination were strikingly higher [26].
The acute, blood pressure-increasing efficacy of Ang II also has a therapeutic benefit. For example, 70% of patients with septic or other shock benefited from it. In them, blood pressure was successfully raised (ATHOS-3 study), accompanied by tachycardia in 8.6% of cases and ischaemia in 4.3% of cases [60].
If Ang II effects persist, hypertension may manifest and contribute to further sequelae such as vascular events, atherosclerosis or progressive end-organ damage. Hypertension development is usually preceded by loss of arterial vascular elasticity [32]. The adaptive immune system and inflammatory responses, together with Ang II and local renin-angiotensin systems, play a key role in the development of Ang II-influenced hypertension. Ang II triggered immune processes, such as increase in inflammatory monocyte numbers, cytokine production or stimulation of adhesion molecules contributing to local accumulation of immune cells, cause inflammatory progression and accelerate hypertension induction. On the other hand, pro-inflammatory factors or immune cells (T lymphocytes, dendritic cells, and macrophages) may also express RAAS components, such as the AT1-receptor, and enhance systemic as well as local Ang II formation, manifesting the involvement of Ang II in the pathogenesis of hypertension. Local ACE/ACE2 imbalance may contribute to RAAS-associated tissue damage [42].
The most significant secondary injuries include vascular and cardiac fibrosis and hypertrophy under the influence of growth factors with the involvement of innate immune responses and inflammatory mediators. Ang II, in addition to receptor-mediated intracellular Ca-release and ROS-formation, leads to Jak2-activation, which induces proliferative and hypertrophic changes in vascular smooth muscle and cardiac myocytes. Jak2 can enhance ROS-formation and thus increase oxidative stress [61].
Endothelial dysfunction is considered an independent, early indicator for the development of cardiovascular disorders. Recently, it was possible for the first time to establish a significant correlation between deterioration of endothelial function (reduced vascular dilatation as a measurable parameter for the presence of endothelial dysfunction) and increasing COVID-19 severity, independent of other factors except age [62]. Thus, the measurement of endothelial dysfunction could become a surrogate marker for persistent endothelial inflammatory responses that cannot be diagnosed with standard laboratory tests.
Progressive impairment of myocardial contractility can develop on the basis of cardiac remodelling, which can manifest clinically as heart failure, tachycardia/arrhythmia or myocardial perfusion disturbance and myocardial infarction. Diabetics, who have been shown to have elevated local Ang II concentrations in the heart, are particularly at risk [32].
Blood coagulation disorders: The link between inflammatory processes and haemostasis, both of which are influenced by the RAAS, has been known for decades and has considerable clinical significance.
In connection with the therapeutic use of Ang II, there is a warning about the risk of arterial and venous thrombotic/thrombo-embolic events (12.9%), which was approximately 2.6 times the risk after placebo. Thrombocytopenia was observed in 9.8% of patients (approximately 1.4 times the placebo value). Thrombo-embolism prophylaxis is recommended to avoid or reduce the risk [60].
Thrombo-embolic complications have been reported in critically ill COVID-19 patients as well as in connection with vaccination. In just under 5 months (until 12 June 2021) since the start of the vaccination campaign, 2778 cases of thrombosis, 1786 cases of embolism/microembolism (including 1639 cases of pulmonary embolism), 90 cases of central sinus vein thrombosis and 983 times of increased bleeding (including 165 cases of immune thrombocytopenia) were reported to the European Medicines Agency (EMA) following vaccination with Comirnaty/Tozinameran. The absolute numbers associated with Vaxzevria were higher (405 cases of sinus vein thrombosis, 2495 cases of pulmonary embolism and 366 cases of immune thrombocytopenia). Qualitatively, however, the spectra were consistent, which is why, contrary to other surveys, no particular specificity can be assumed for Vaxzevria; the side-effect spectrum of spike-inducing vaccines exhibits class specificity [27,63].
A similarity with auto-immune Heparin-Induced Thrombocytopenia (aHIT) has been postulated. However, this hypothesis is not unique in view of the influence of increased Ang II concentrations on important blood coagulation factors. Several findings have shown that Ang II, with the participation of IL-6, stimulates the synthesis of the tissue factor (factor III) which, together with proconvertin in the subendothelial space, dominates the extrinsic pathway of blood coagulation. After initiation of the coagulation process and the activation of inflammatory molecules, endothelial cells and monocytes produced more tissue factor, especially in cytokine-activated endothelial cells. RAAS inhibitors (ACEI, ARBs, renin inhibitors), on the other hand, down-regulated the synthesis of tissue factor or reduced its activity, supporting pro-coagulatory effects of an activated RAAS and confirming the interaction between endothelial inflammation and coagulation.
Circulating Ang II increased thrombin formation, the key enzyme in plasmatic coagulation and important for platelet activation. In contrast, ACEI treatment inhibited thrombin formation in hypertensives and this was found to be clinically relevant in the reduction of thrombo-embolic events after ACEI treatment in high-risk patients independent of blood pressure reduction [31].
Through its receptor, Ang II (and Ang IV) stimulated the expression or production of fibrinolysis-inhibiting Plasminogen Activator Inhibitor 1 (PAI-1) in endothelial cells and vascular smooth muscle cells, sensitised platelets, promoted superoxide radical production and induced tissue factor expression; in contrast, AT1R Blockers (ARBs) improved fibrinolytic parameters in patients and ACEI improved fibrinolytic balance [31,39].
Recently, it has been shown that platelets possess ACE2 so that SARS-CoV-2 and spikes can act directly by binding to their receptor. Thus, spikes are involved in thrombus formation as well as in inflammatory reactions by releasing coagulation and inflammatory factors. Platelet aggregation and ATP release are promoted in a dose-dependent manner [64].
Up-regulation of AT1R in platelets has been described in hypercholesterolaemia, which could be clinically relevant when combined with SARS-CoV-2 infection or spike production after vaccination [38].
In 2021, cell fusion was recognised as a link between SARS-CoV-2 spike protein, COVID-19 complications or/and vaccination side effects, especially as a trigger of the blood coagulation cascade [17]. The preconditions discussed for this are that syncytia express the tissue factor TF that contact with blood is given, which is present in endothelial cells, and that tenase activity is sufficient to initiate the coagulation cascade. Viral fusogens are able to form large syncytia that tend to die, expose the thrombogenic basement membrane when detached and support platelet-dependent coagulation in this way. Syncytia formation can occur between infected, virus-replicating cells and healthy neighbouring cells, but also through extracellular spike-vesicles that connect healthy cells like bridges ("fusion from the outside"). This would explain cases of thrombosis in non-infected tissue.
The spike/syncytia-triggered coagulation cascade not only mediates blood coagulation, but also interacts with signalling pathways that regulate inflammation, fibrosis and several other COVID-19-associated conditions [17].
Nervous system disorders: Nervous system disorders were among the most common organ-related systemic spike-based vaccine adverse events (about 16%-20%) and were at least as dangerous as cardiovascular adverse events in terms of fatal outcome [28]. Headache dominated (up to 6 digits), followed by dizziness (up to 5 digits), paraesthesias/sensitivity disorders (up to 5 digits), attention and balance disorders (up to 4 digits), facial nerve palsy (up to 4 digits) and other disorders. Vasospasms triggered by vasoactive substances such as catecholamines or Ang II are possible causes of stabbing, flash-like headaches (analogous to reversible cerebral vasoconstriction syndrome-RCVS). Associated with SARS-CoV-2-infections as well as spike-based vaccinations, there are initial reports supporting a vasoconstrictive genesis, possibly with ischaemic sequelae [65,66]. The inducing of dizziness/balance disorders can also be based on an impairment of the blood supply to the sensitive vestibular organ, but can also be an indirect consequence of blood pressure fluctuations or cardiac arrhythmias. Decreased blood and oxygen supply, as well as direct neuronal disturbance by S1-spike-proteins, can lead to central performance deficits that require further clarification. An insufficient blood supply (vasoconstriction, vasculitis) via the smallest nerve-supplying blood vessels provoked by ACE2-downregulation is equally debatable as a cause of bothersome and debilitating sensations. Auto-immunological processes can also be considered pathogenetically. Longer-term dysregulations of central performance are obvious on the basis of the experimental findings described above.
In addition to the involvement of vasoconstriction, the fusion of neurons or the fusion between neurons and glial cells is discussed with regard to the triggering of neurological symptoms [17]. This could explain neuropathic pain that lasts for months. The prerequisite for central nervous cognitive disorders is that spikes have been detected in the brain, which is the case.
The brain has its own independent local RAAS. It is significantly involved in blood pressure regulation by influencing fluid homeostasis and sympathetic activity; water retention is enhanced by the release of vasopressin after Ang II stimulation. Due to the low expression of AT1R in parenchymal arterioles, it is suggested that there is therefore less likelihood of focal ischaemia associated with a stress-induced Ang II increase [67].
Among other things, a neuronal, AT1R-mediated ACE2 downregulation with simultaneous ADAM17 upregulation has been described, which could provide a highly significant explanation for the triggering of cardiovascular responses by COVID-19 disease, as well as for adverse side effects of spike-based vaccines, if these findings can be clinically corroborated [32].
The role of the central RAAS in the development of cognitive dysfunction, dementia and Alzheimer's disease should also be noted with attention. Thus, increased neuronal and perivascular activity of ACE and Ang II was found in vessels of the parietal and frontal cortex of Alzheimer's patients; chronic Ang II activity lowered cognitive functions, reduced synaptic plasticity and promoted neuro-inflammation in animal experiments [40]. Ang II(3-8; fragment of Ang II) has been known to affect cognitive, motor and sensory functions via its AT4 receptor [68]. The finding of AT1R-triggered Nox2-dependent ROS production in perivascular macrophages, which led to cognitive dysfunction, seems worth mentioning [32].
The accumulation of ß-amyloid could be enhanced by Ang II, whereas plaque formation could be prevented by AT1R deletion in animal experiments [32]. Thus, independent of a blood pressure effect, a neuroprotective efficacy of inhibited AT1-receptors can be assumed. Blood-brain-barrier-compatible ACE inhibitors were able to improve cognitive deficits in the elderly, also independently of blood pressure reduction, possibly because of their anti-inflammatory effects. Angiotensin Receptor Blockers (ARBs) appear to be therapeutically effective in early AD. They appear to have preventive anti-neuroinflammatory effects and protect against ischaemia-induced outcomes, such as ischaemic stroke. In addition, evidence exists for neuroprotective effects in Parkinson's disease [32,40]. Ang 1-7 has also been shown to be neuroprotective (improved endothelium-dependent relaxation, reduction in infarct size and neurological deficits, anti-inflammatory) [1].
Muscular disturbances: The sceletal musculature possesses the spike receptor enzyme ACE2 in addition to the endothelial vascular ACE2, so that a direct influence by spikes is not surprising [69]. Muscular disorders are therefore among the frequent sequelae of COVID-19 disease as well as the side-effect spectrum of spike-based vaccination (incidence of myalgia in the 6-digit range) [28].
Ang II/AT1R are also the most essential player in the signaling chain mediating the development of muscle atrophy. Ang II induces intracellular protein degradation and selective myosin loss through increased expression of proteasome proteolysis. The catabolic Ang II effects are thought to be enhanced during cachectic states and by malnutrition [70]. Ang II can thus lead to proteolysis of skeletal muscle and inhibit the proliferation of satellite cells important for muscle cell repair. These findings provide sufficient rationale for a causal relationship between the occurrence of muscle atrophy and rhabdomyolysis after spike-based vaccination (frequency in the 2-3 digit range in each case) [28]. Auto-immune processes should also be considered as causation (see next section). AT2R stimulation has the opposite, muscle-regenerating effects.
Auto-immunological disorders: The involvement of the RAAS, or more precisely Ang II and ACE2, in triggering auto-/immunological responses has received little attention for a long time. ACE2 causes both spikes and SARS-CoViruses to influence these responses in an equal manner. Already in 2009, it was shown that Ang II activity inhibition (ACEI, ARBs, and renin inhibitors) led to significant improvement or prevention of experimentally triggered autoimmune encephalomyelitis (MOG-EAE) [71]. Via modulation of T-cell functions (Th1/Th17, CD4-T), Ang II/AT1R is involved in auto-immune processes such as multiple sclerosis, autoimmune uveitis or encephalomyelitis and myocarditis. In addition to microglial activation and cytotoxic T-cell infiltration, an overstimulated immune response and extensive neuroinflammation were found in autopsy tissue from neurological COVID-19 sufferers [5]. ACEI/ARBs, on the other hand, inhibited antigen-specific T cell activation, suppressed cytokine release by Th1 and Th17 and induced regulatory T cells (Treg) [40].
Since 2021, the occurrence of inflammatory rheumatic diseases (e.g. rheumatoid arthritis, polymyalgia rheumatica PMR, giant cell arteritis, reactive arthritis, lupus, Sjögren's syndrome) in the context of COVID-19 disease has been observed repeatedly. These have been hypothesised to have an auto-immunological pathogenesis [72-74]. Although there are similarities between COVID-19 associated endothelial inflammatory response and systemic vasculitis or PMR, the dysregulation of the RAAS causally involved in inflammatory responses has been ignored [75].
The same applies to the rheumatic complaints observed after vaccination, which have been reported several times. Within a few days up to 14 days after vaccination, typical polymyalgia rheumatica cases (new onset or recurrence) have been described, without considering the involvement of impaired RAAS/Ang II homeostasis [76-80].
Very early after the start of the COVID-19 vaccination campaign (December 2020 to February 2021), it was noticed that Immune-Mediated Diseases (IMD), particularly autoimmune rheumatic in nature (21 out of 27 cases) occurred in temporal association with the 1st or 2nd dose of vaccination (onset after approx. 4 days on median) [81]. 6 affected persons suffered from either myasthenia gravis (n=2), MS (n=1), neurosarcoidosis (n=1) or pericarditis (n=2). In some cases, an assiociation with vaccination could not be ignored. For most of the observed inflammatory reactions, the authors suspected that non-specific effects of the vaccine adjuvants were causally involved. In some cases of vasculitis associated with skin eruptions, an upregulation of Interferon-Stimulated Genes (ISGs) triggered by TLR-7/9 was discussed.
Among 27 hospitalised patients who developed IMDs within 3 weeks, mostly already after the first COVID-19 vaccine dose, were cases of PMR and giant cell arteritis (n=16), necrotising myositis, acute rhabdomyolysis, systemic vasculitis, immune thrombocytopenic purpura, rheumatoid arthritis, anti-synthetase syndrome or Stills disease. IMDs occurred in principle independently of the vaccine type, but it was suspected that the adjuvant properties of mRNA vaccines might lead more frequently to Th17 inflammatory reactions and effects on the immune system. Genetic predisposition has also been suggested as a risk factor for the occurrence of IMD. Due to the lack of or only minor responses to revaccination (n=11), partly during treatment of IMD, and despite a striking accumulation of rare and severe diseases, such as giant cell arteritis or rhabdomyolysis, the investigators rejected a causal relationship between vaccination and IMD, but recommended an adjustment of vaccination policies to the weakening SARS-CoV-2 infections [82].
A broad spectrum of auto-immunologically caused neurological diseases (new-onset cases: VITT with CVST n=3; CNS demyelinating diseases n=12; inflammatory peripheral neuropathies n=4; myositis n=2; encephalitis n=1; giant cell arteritis n=1; new episode of an already existing disease: MS n=2; myositis n=1; myasthenia n=1), which was observed 1-4 weeks after COVID-19 vaccinations, made inpatient treatment of the affected persons necessary [83]. Some of the symptomatics had already been noticed and known as a consequence of a SARS-CoV-2 infection. The cause of the simultaneous presence of painful joints and skin florescences after COVID-19 vaccination turned out to be vasculitis of small capillaries with IgA deposits, which was confirmed bioptically [84]. The authors recommended that spike-based COVID-19 vaccination be included in the causative list of an IgAV. Commentators corroborated this approach and pointed to other systemic vasculitis cases associated with COVID-19 vaccination that also involved larger vessels [85]. In addition, a considerable number of auto-immune/inflammatory cases (n=19) were reported, which included several polymyalgia rheumatica cases (n=5). The pathogenetic mechanism leading to vasculitis and other systemic autoimmune phenomena requires further clarification, according to the authors.
Regulatory T cells (Tregs) prevent immune activation and the development of autoimmune diseases, in the case of RAAS activation Ang II-induced hypertension, endothelial dysfunction, and vasculitis and ROS production.
The obvious spike (S1)/ACE2 interaction was neither addressed nor clarified in terms of causation.
Vascular disorders: The common characteristic of many spike-induced symptoms are undoubtedly macro- and microvascular disturbances with corresponding consequences. At the beginning there is the spike/ACE2 interaction in the vascular system with subsequent dysregulation of the RAAS, which can lead to vasoconstriction (see also under 2.: Ang II mediated vasoconstriction) and/or vasculitis or vasculopathy.
As already mentioned several times, endogenous Ang II favours pro-inflammatory reactions in its target organs, especially in the vessel wall. Ang II is significantly involved in the process of development of severe vasculopathy [86]. A self-sustaining positive feed-back mechanism between upregulation of Ang II blood vessel tone and vasculitis is discussed [87]. Mononuclear cells, such as T cells, monocytes and macrophages, play an important role. Dendritic cells of the immune system were activated by Ang II, T cells proliferated in response to Ang II.
Immune cells and pro-inflammatory cytokines are involved in the expression of vasoconstriction and ROS production, endothelial dysfunction, hypertension, arterial wall stiffness/arteriosclerosis, aneurysm development, ageing processes, cardiac hypertrophy and fibrosis, renal fibrosis and microbiome function as a component of Ang II signal transduction mechanisms. Activation of the cytoplasmic transcription factor NF-ĸB, which controls a network of chemokines, multiplies the effects of Ang II; inhibition of NF-ĸB blocked some of it (IL-6, adhesion molecules, chemokines), prevents inflammatory changes and ameliorates cardiac and renal damage in a model with high Ang II levels [86]. NF-ĸB is inducible by lipid oxidation, IL-1, TNF and infectious agents. Its role in triggering the inflammatory vascular cytokine cascade towards atherosclerotic progression is thus manifested [87].
For some time, vasculitis has been considered an independent risk factor for the development of atherosclerosis. The known pro-inflammatory and pro-atherogenic Ang II effects modulate and amplify the production of ROS with subsequent NO inactivation, the production of cytokines (IL-6) and adhesion molecules. A synergism was found between hyperlipidaemia and Ang II. The importance of the cytoplasmic transcription factor NF-ĸB has already been pointed out in connection with the development of vasculitis.
In contrast, vasoprotective, anti-atherogenic effects of ACE inhibitors as well as of AT1R blockers could be demonstrated experimentally, in part also clinically [87].
Renal disorders: Ang II is known to affect the kidneys, which have a complete local RAAS, in a fundamental way; it exerts pro-inflammatory effects after chemokine expression through an NF-ĸB-dependent mechanism, reduces renal blood flow, stimulates water and salt reabsorption and aldosterone secretion, and contributes significantly to the progression of diabetic nephropathy [88]. Ang II induced renal inflammation and fibrosis significantly lead to chronic renal failure [32]. A dysregulated immune response is involved in pathological tubular, glomerular and vascular disorders; ACE2 is particularly expressed in the proximal tubule and glomerular endothelial cells [5]. Counteracting effects are produced by the AT2-receptor and the ACE2/Ang(1-7)/MAS axis [41,89]. The AT2-receptor, expressed in vascular and tubular elements, mediates natriuresis supported by dopamine1-like receptors. It protects against ischaemic-inflammatory tissue destruction [41].
Increased AT1R expression on immune cells appears to counteract direct pathogenic renal AT1R effects [43].
Other consequences of persistently high RAAS activity, such as the influence on energy homeostasis, the modulation of insulin action and glucose metabolism, the influence on adipocyte hypertrophy and adipogenesis, the triggering of an inflammatory response in adipose tissue [87], carcinogenesis or the involvement of RAAS activity in ageing processes require a separate topic [90].
Diagnostic consequences and therapeutic options
Due to the high complexity of the RAAS, a detailed differential diagnosis is required to limit or cure spike-induced side effects.
First of all, reactions to excipients, stabilisers, solubilisers and/or other admixtures of the finished drug should be ruled out. If spike-relevant symptoms are present, a comprehensive cardiovascular differential diagnosis should be performed, including testing of endothelial dysfunction and regional sequelae of possible vasoconstriction or cytotoxic tissue injury, an examination of the coagulation status and the immune system as well as the regional presence of vaccine spikes and generally of markers of the RAAS (Ang I/II, AT1R, AT2R adrenalin/norepinephrine, Ang 1-7, MAS, ACE, ACE2, chymase, renin, aldosterone, antibodies-systemic and/or local, possibly on cells of the immune system and platelets). This diagnostic spectrum should also be taken into account appropriately when clarifying fatal outcomes associated with vaccination.
The following aspects can be helpful in interpreting the results:
Counter-regulatory or competitive mechanisms: binding of spike S1 subunit in competition with the physiological ligands Ang I/II on the ACE2 enzyme, Ang II activates both AT1R and AT2R; AT1R antagonises AT2R; AT2R can directly inhibit AT1R, high Ang II concentrations are limited by renin suppression; antibodies can reduce Ang II effects, ACE2 counter-regulates an activated RAAS; Ang 1-7 effects oppose those of Ang II, Ang 1-7 decreases affinity of Ang II to AT1R; ACE2/Ang1-7/AT2R/MAS axis opposes Ang II/NA/AT1R axis, local modulation of systemic RAAS effects, AT1R on immune cells has protective properties with respect to pathogenic renal AT1R-induced injury, downregulated ACE2 reduces viral infectivity, but may increase side effect inducing, regulatory T cells prevent immune system activation.
Reinforcing mechanisms in the RAAS: Ang II initiates NA release and reuptake inhibition; formation of Ang 1-7 by ACE2 and also by PRCP, POP, NEP; simultaneous blockade of membrane-bound and soluble ACE2 by SARS-CoV-2; ACE2 and MAS complement each other in protective function against Ang II; positive feedback mechanism between up-regulation of Ang II vascular tone and vasculitis; Nox-generated ROS activates AT1R, stimulates other ROS-producing systems and multiplies oxidative stress; pro-inflammatory factors or immune cells may enhance RAAS components or/and local Ang II formation.
Multiple functions of the interacting components: Spike S1 subunit (RBD) acts as antigen, active agent and fusion mediator; ACE2 is receptor of Ang I and II, of S1 spikes, a fusion mediator and counterpart in the RAAS.
Consideration of genetic polymorphism: (A1166C des AT1R) and possible desensitisation (acute efficacy of Ang II on AT1R (enhancement) differs from chronic (attenuation)).
Consideration of individual conditions of the recipient:
ACE2 downregulation or loss of function: SARS-CoV-2 infection, older age, receptor occupation with non-neutralised vaccine spikes, auto-antibodies against ACE2; in diabetes, hypertension, stress, cardiac hypertrophy and fibrosis, after myocardial infarction, in conditions with rising Ang II levels/AT1R activity, in H1N1 and H5N1 infection, ovariectomy or in association with oestrogen receptor blockers, in people with blood group A.
Upregulation of ACE2: Treatment with ACEI or ARBs; COPD; aortic stenosis in older age (soluble ACE2); inflammatory cytokines in severe COVID-19; smoking.
Increase in myocardial ACE2 concentrations: Pretreatment with ACEI and ARBs.
Older age: Increased POP/PCRP- and TMPRSS2-activity; ACE2 loss/reduction in function; increased chymase activity with markedly increased Ang II-concentrations in cardiac tissue and poor response to ACEI; inverse correlation between age and RAAS activity.
Adults: AT1R is more strongly expressed in the immune system than AT2R.
Children: Reduced ACE2 expression.
RAAS activation: By hypovolaemia, hypotension, salt restriction, stress.
AT1R stimulation: Pro-inflammatory factors or immune cells, gluco-/mineralocorticoids, LDL, hypercholesterolaemia in men, insulin, insulin-like growth factor, progesterone, erythropoietin, bradykinin B2-receptor; up-regulation in platelets in hypercholesterolaemia.
AT1R inhibition: Interferon-y, retinoic acid, vitamin A, oestrogen, HMG-CoA-reductase inhibitors, epidermal growth factor EGF, PDGF, thyroid hormone, NO, forskolin; interaction with β-receptors; various pathophysiological conditions, such as sepsis.
Activation of AT2R expression: under conditions with tissue or vascular damage, in myocardial infarction, heart failure, renal failure or cerebral ischaemia, peripheral nerve disturbances; in inflamed tissue, in cells of the innate and adaptive immune system in rheumatoid arthritis; female sex.
AT2R downregulation: Ang II, NA, growth factors, glucocorticoids; vascular musculature of hypertensives, bladder cancer tissue.
Loss of MAS function: Impaired cardiac function.
Increased POP/PCRP activity: Obesity, cardiovascular risk factors, atherosclerosis/unstable plaques, renal disease, inflammation, and diabetes.
COVID-19 disease: Increase in auto-antibodies against ACE2 or Ang II; In severe cases: correlation between high Ang II levels/ACE2-deficiency and COVID-19 severity; AT1R down-regulation with non-response to Ang II.
Heart failure: Increase in soluble ACE2 (inverse correlation with degree of cardiac dysfunction).
African-Americans: Increased TMPRSS2 activity.
In conditions of low baseline ACE2 levels (older age, increased TMPRSS2 activity in older age, comorbidity, stress), any additional ACE2 downregulation, including iatrogenically caused ACE2 downregulation, can be dangerous, requiring rapid intervention. A combined failure of ACE2 and MAS may exacerbate the situation. On the other hand, increased PRCP, POP and/or NEP activity with intensified conversion of Ang II into Ang 1-7, e.g. in older age, can have a compensatory effect, but not in younger people who lack this potential.
Effects may change with chronic progression and sustained high Ang II-efficacy, effects may even reverse depending on tissue specificity (e.g. triggering of protective effects of AT1R activation). The numerous counter-regulatory or reinforcing actions of RAAS components and their multiple functions must be taken into account, as well as the specifics of the target organ, the gender-typical characteristics of AT1R activity, age-specificity, previous treatment or existing tissue damage.
In conditions of low baseline ACE2 levels (older age, increased TMPRSS2 activity in older age, comorbidity, stress), any additional ACE2 downregulation, including iatrogenically caused ACE2 downregulation, can be dangerous, requiring rapid intervention. A combined failure of ACE2 and MAS may exacerbate the situation. On the other hand, increased PRCP, POP and/or NEP activity with intensified conversion of Ang II into Ang 1-7, e.g. in older age, can have a compensatory effect, but not in younger people who lack this potential.
Effects may change with chronic progression and sustained high Ang II-efficacy, effects may even reverse depending on tissue specificity (e.g. triggering of protective effects of AT1R activation). The numerous counter-regulatory or reinforcing actions of RAAS components and their multiple functions must be taken into account, as well as the specifics of the target organ, the gender-typical characteristics of AT1R activity, age-specificity, previous treatment or existing tissue damage.
In contrast to countless clinically relevant publications on SARS-CoV-2 infection and its treatment or prevention, there is a large gap in the field of systematic recording and treatment of vaccination side effects. Their possible causation is not communicated adequately or only in selected (individual) cases, but usually without reference to an underlying dysregulated RAAS. Those who are confronted with the unusually extensive and unexpected consequences of vaccination are therefore left unenlightened and often at a loss. This analysis attempts to reduce this gap.
Considerable evidence suggests that both restoration of impaired ACE2 function and inhibition of activated, dysregulated RAAS should be able to attenuate or eliminate much of the resulting adverse experiences. The efficacy of RAAS inhibitors has been repeatedly demonstrated. The following drug treatment can be considered:
• Soluble recombinant ACE2 is in the clinical testing phase; it replaces functionally impaired ACE2 and stimulates protective RAAS components.
• ACE Inhibitors (ACEI) with well-founded experience since the 1980s; they reduce the formation of Ang II, but not that caused by chymase (loss of efficacy!) and they inhibit the inactivation of the vasodilator tissue hormone bradykinin.
• Renin inhibitors have been approved since 2007. They prevent the formation of Ang II, but do not inhibit the breakdown of bradykinin. A comparative analysis between ACEI and renin inhibitors showed no essential differences in mortality and side effects [91].
• Angiotensin Receptor Blockers (ARBs) have been available since about 1995. Due to their mode of action-selective inhibition of Ang II action at the AT1-receptor-they are particularly effective when Ang II and AT1R activity are high. Their effect is supported by the Ang II triggered activation of AT2R with favouring of counter-regulatory mechanisms (vasodilation/blood pressure reduction, tissue protection and inflammation inhibition, inhibition of aldosterone synthesis, especially reduction of myocardial fibrosis after acute myocardial infarction, suppression of pro-inflammatory cytokines, remodelling reduction, improved lipid metabolism, upregulation of endothelial NOS production, increased adiponectin levels, improvement of endothelial dysfunction, reduced production of adhesion molecules, anti-apoptosis; [31,87]. ACE2 expression of various tissues is increased [9,92]. They do not affect vasodilator kinins and the AT2-receptor. The advantages of ARBs, including their high tolerability, are convincing. The onset of action, which is not immediately apparent, must be taken into account.
In the case of dominant vasoconstrictive symptoms (e.g. blood pressure crises, ischaemia, sudden stabbing headaches, etc.), the use of vasodilators (e.g. calcium antagonists) or anti-catecholaminergic drugs should be considered additionally or alternatively. Special diagnostics and therapy are required for immunological/auto-immunological, metabolic, reproductive organ and blood coagulation disorders as well as in cancer.
A number of hypothetical treatment options are discussed. These include AT2R-agonists and Ang 1-7 analogues or dapagliflozin with possible ACE2-increasing effects [93]. Direct spike blockade or/and prevention of spike-induced cell fusion or/and inhibition of spike/ACE2-interaction appear useful as long as spike production persists in the organism and is detectable. Examples are:
• Administration of specific antibodies against the RBD of the SARS-CoV-2 spike protein (CR3022, LY-CoV1404, casirivimab, Imdevimab) or spike-neutralising antibodies, such as LY-CoV555 (Bamlanivimab) [93].
• Inhibition of cytotoxic cell fusion by niclosamide, clofazimine, salinomycin, also by nitazoxanide, hexachlorophene, dichlorophene and others [18].
• Binding or blocking of the spike protein (ivermectin, fisetin, apigenin, rutin, silymarin, Prunella vulgaris) [94].
• Blockade of the spike/ACE2-interface (N-acetylcysteine/NAC) [94].
• Influence on or inhibition of the spike/ACE2-interaction (quercetin, emodin, curcumin, dandelion extract, cloves) [94].
• Spike degradation (nattokinase) [94].
The therapy of undesirable consequences of spike-inducing vaccination is and remains a challenge. Knowledge and assessment of the influence of relevant factors of the complex RAAS and the Ang II-specific effects are the basis for successful therapeutic intervention. An individualised approach depending on symptoms and differentiated diagnosis is essential.
Analysis of the scientific literature provided convincing evidence of the adverse effects of pathophysiologically harmful activation of the ReninAngiotensin-Aldosterone System (RAAS). RAAS activation also occurs as a consequence of the high-affinity spike/ACE2 interaction. This explains the class-specific side effects of spike-inducing vaccinations.
RAAS-activation is not limited to acute and long-term injury of the cardiovascular system (heart and vessels), but also affects other vital organs such as the kidneys, the nervous system or the musculoskeletal system and is involved in triggering blood clotting disorders, inflammatory and autoimmunological reactions, tissue fibrosis and proliferation etc.. The disorders caused by an activated RAAS often manifest first in the blood vessels (vasoconstriction, vasculitis, endothelial dysfunction, vasculopathy, coagulation disorders, atherosclerosis); their supply area may be affected.
RAAS-activation may be caused by impairment of counter-regulatory influences, such as down-regulation of ACE2. ACE2 is responsible for the degradation of Ang I/II and is thus an important modulator in limiting the harmful consequences of an increase in Ang II concentration.
In competition with natural ligands, SARS-CoViruses impair hydrolytic ACE2 activity, which is the basis for their broad spectrum of organ disturbances. Similarly, non-neutralised vaccine spikes (RBD of the S1-subunit) can lead to organ dysfunction via down-regulation of ACE2 and resulting RAAS activation. The consequences of an activated RAAS with increased angiotensin II concentrations must therefore be considered for the class-specific side effect spectrum of spike-inducing vaccines in any case. The recently discovered cytotoxic cell fusion complicates the spike-induced symptomatology.
There is no doubt that pathological hyperactivity of the reactive RAAS is undesirable and harm prevention can only be achieved by consistently refraining from any unfavourable influence. However, spike-inducing vaccines, due to their mode of action, enhance RAAS activation, which makes them unsuitable as an invasive preventive intervention in previously healthy individuals. Harm reduction can never compete with the primacy of harm prevention. Ignoring this principle and failing to provide relevant information has led to an unprecedented explosion of unexpected, sometimes life-threatening, side effects from these vaccines.
The diagnostic evaluation of symptoms or side effects associated with the use of spike-inducing vaccine and their treatment is challenging. A number of factors need to be assessed, and numerous counterregulatory, sometimes reinforcing influences, multiple functions of RAAS-components as well as a considerable number of individual conditions (age, sex, local tissue specifics, comorbidity etc.), need to be taken into account.
The hypothesis presented concerning spike-induced side effects provides also a rationale to mitigate harm with RAAS-inhibitors, preferably ARBs. A few experimental drugs are under discussion. However, due to the complexity of the RAAS, it will be difficult to predict with certainty the success of treatment.
Clinical trial results to demonstrate evidence are urgently needed, but targets, inclusion and exclusion criteria must be fine-tuned behind the background of current knowledge.
Only a limited selection of health disorders or side effects associated with the spike/Ang II-efficacy is included in this analysis. Clinical study results to confirm and prove evidence are urgently needed.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lehmann KJ SARS-CoV-2-Spike Interactions with the Renin-Angiotensin-Aldosterone System-Consequences of Adverse Reactions of Vaccination ''J Biol Todays World, 2023,12(4), 001-013
Received: 17-Jul-2023, Manuscript No. JBTW-23-106542; Editor assigned: 20-Jul-2023, Pre QC No. JBTW-23-106542 (PQ); Reviewed: 03-Aug-2023, QC No. JBTW-23-106542; Revised: 10-Aug-2023, Manuscript No. JBTW-23-106542 (R); Published: 17-Aug-2023, DOI: 10.35248/2322-3308-12.4.004
Copyright: © 2023 Lehmann KJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.