Research Article - (2021) Volume 3, Issue 1
Oral Squamous Cell Carcinoma (OSCC) is a common malignant head and neck tumor. The mainstay treatment of OSCC is surgery-centered multimodality therapy. Reconstructive surgery is always needed to restore the oral function after ablative surgery. Role of NACT in the treatment of head and neck cancers is still controversial. In this study, we retrospectively reviewed a group of patients with locally advanced but resectable OSCC in a single cancer center. A total of 32 patients who received NACT followed by surgery and 35 patients who underwent surgery without NACT were included in this study. No statistically significant result noted within demographic characteristics, pathology differentiation, site except buccal mucosa (p value=0.04), clinical T4a stage, N stage, positive surgical margins, mandibular preservation rate, skin and RMT involvement and facial edema. 12 out of 32 (37.5%) patients who received NACT were good responders including two patients (6.2%) had a Complete Response (CR) and 20 patients (62.5%) had a PR. NACT doesn’t influence on choice of surgery because all patients had undergone radical surgery. Kaplanâ €“Meier analysis showed no statistical difference between two groups (p-value: 0.159) with no difference in hazard function, which remain 1.00 at 1-yr. Patients in the non-NACT group had a statistically better RFS (median 11 months with 95% CI 9.17-12.82) than the patients in the NACT group (median 8 months at 95% CI 6.42-9.57). So, if OCSCC is resectable than resect primarily.
Oral cavity cancer • Locally advanced • NACT • Resectable
Oral Squamous Cell Carcinoma (OSCC) is a common malignant head and neck tumor with approximately 300,000 new cases worldwide per year. The mainstay treatment of OSCC is surgery-centered multimodality therapy. Reconstructive surgery is always needed to restore the oral function after ablative surgery.
Following the advances in imaging and therapies, the prognosis of patients with OSCC has improved, with its 5-year survival rate arriving at about 70% recently [1].
Neoadjuvant Chemotherapy (NACT) refers to chemotherapy administered before surgery. Its role in the treatment of head and neck cancers is still controversial [2]. Two main concerns for the application of systemic therapy are the improvement of long-term survival and organ/ function preservation. Induction chemotherapy (before radiotherapy) was firstly started in laryngeal cancer in order to select appropriate patients for organ preservation therapy. Then, questions were asked whether an addition of chemotherapy before surgery or radiotherapy could improve survival. The answers were still not clear even after a lot of studies including randomized clinical trials and meta-analysis [3-5].
In addition, there are still few studies investigating the influence of NACT on the following surgery. Can we perform a limited surgery after NACT? Will it affect the surgical margins and prognosis? In this study, we retrospectively reviewed a group of patients with locally advanced but resectable OSCC in a single cancer center.
This was a retrospective and observational study. The study was approved by the institutional review board at Sri Aurobindo Institute of Medical Science, Indore. Written informed consent was obtained from all patients at their first visit. We comprehensively reviewed the clinical records of OSCC patients who were treated in our institute during October 2018 to June 2020.
Finally, a total of 32 patients who received NACT followed by surgery and 35 patients who underwent surgery without NACT were included in this study.
Inclusion criteria
• Oral cavity SCC diagnosed with biopsy (punch/ incisional)
• Locally advanced disease T4aNanyMo
• Treatment Naive patients (Doesn’t received prior radiation/surgical/ chemotherapy)
• Good performance status ECOG 0-1
• T4b including only low ITF or masticator space involvement
Inclusion criteria
• Metastatic disease
• Poor performance status who was not fit for either NACT or surgery
• High ITF (supra-notchal disease involvement)
Staging workup included indirect laryngoscopy, tumor biopsy, and computed tomographic imaging. The site and extent of the tumors were evaluated by clinical examination and imaging scans.
Treatments
Neoadjuvant chemotherapy: The patients in the NACT group received two-three cycles of preoperative NACT. TPF regimen (docetaxel 60 mg/m2 intravenously on day 1, followed by cisplatin 75 mg/m2 intravenously on day 1, followed by fluorouracil 750 mg/m2 per day as a 120-hr continuous intravenous infusion on days 1 through 5) was administered every 3 weeks. Supportive measures including dexamethasone, antiemetics, and hydration/diuretics were also administered. Primary prophylaxis with recombinant granulocyte colony-stimulating factor was prescribed 48 hrs after the chemotherapy. Chemotherapy dose reductions were allowed for grade 3–4 hematologic, GI or renal toxicities occurring after cycle one. Another regimen is 5-FU and Cisplatin only.
Surgery: Radical surgery with flap reconstruction done in almost all groups of patients. In the non-NACT group, upfront radical surgery was performed. Neck dissection and flap reconstruction were also done. In the NACT group, the choice of conservative or radical surgery is not merely determined by the response of NACT. Multiple aspects patient’s clinical manifestations were also considered, such as primary site of tumor, pathology differentiation, T and N stages, and shape of tumor. Surgery was performed at least 3 weeks after completion of NACT. The surgical extent is partly determined by the response after NACT. For radical surgery with flap reconstruction, wide excision of the primary lesion with 1-cm surgical margin and neck dissection were performed. Microvascular free flap or pedicled pectoralis major flap were used to reconstruct the defects after the surgery.
Postoperative radiotherapy: Adjuvant treatment after surgical extirpation was determined on the basis of standard PORT criteria, including extracapsular spread, positive margins, regional metastasis, and perineural and lympho-vascular invasion. Postoperative radiotherapy was initiated within 4–6 weeks after surgery for the patients with adverse features. Standard conformal or intensity-modulated radiotherapy was administered at a dose of 2 Gy per day, 5 days per week, for 6 weeks (60 Gy in total). Concurrent cisplatin given according to high-risk features in HPE report.
Assessments: The stage of OSCC was evaluated according to the UICC TNM (8th edition). Clinical tumor response was evaluated 3 weeks after NACT by physical examination and imaging scan. The standard World Health Organization (WHO) RECIST1.1 criteria were used to evaluate the response after NACT. Patients with a response of at least 50% (more than partial response, PR) were classified as good responders. Toxicities were assessed weekly during and after completion of NACT according to Common Terminology Criteria for Adverse Events (CTCAE, version 4.0). Surgical margins were evaluated pathologically by post-operative HE staining section.
Follow-up and outcomes: All the patients were followed up for at least 5 years, monitored every 1 month during the first year every 3 months in second year and every 6 months during the subsequent 3–5 years, and once per year thereafter until death. The primary outcomes of interest were Disease-Specific Survival (DSS), Disease-Free Survival (DFS). DSS was defined as the time from treatment to the time of death from OSCC.
Statistical analysis: For descriptive analysis, categorical data were expressed as number and percentage. The baseline data in the two subgroups were compared by the Chi-square test to assess differences among the clinical variables. The Kaplan–Meier method and the log-rank test were used to assess differences in the survival between different treatment subgroups. IBM SPSS statistics software was used to perform the statistical analysis. A two-tailed P<0.05 was considered statistically significant.
Among 67 patients enrolled in this study, 35 patients received upfront radical surgery (non-NACT group) and 32 patients received NACT followed by surgery (NACT group). The baseline data of the two groups were shown in Table 1.
| Parameters | Non-NACT | NACT | Total | P-value |
|---|---|---|---|---|
| Sex | 1 | |||
| Male | 22 | 23 | 45 | |
| Female | 13 | 11 | 24 | |
| Age | 0.41 | |||
| <40 | 10 | 10 | 20 | |
| 40-60 | 22 | 18 | 40 | |
| >60 | 3 | 4 | 7 | |
| Pathology differentiation | 0.56 | |||
| Well- | ||||
| Moderate- | 29 | 28 | 57 | |
| Poor- | 5 | 2 | 7 | |
| 1 | 2 | 3 | ||
| Site | ||||
| Buccal Mucosa | 13 | 13 | 26 | 0.04 |
| Alveolus | 8 | 7 | 15 | 0.32 |
| GBS | 7 | 5 | 12 | 0.51 |
| Tongue | 5 | 4 | 9 | 0.58 |
| Lip | 2 | 1 | 3 | 0.54 |
| Maxilla | 0 | 2 | 2 | 0.45 |
| T | ||||
| 0 | 0 | 2 | 2 | 0.041 |
| 1 | 0 | 1 | 1 | 0.045 |
| 2 | 0 | 2 | 2 | 0.48 |
| 3 | 0 | 3 | 17 | 0.43 |
| 4a | 35 | 24 | 59 | 0.56 |
| N | ||||
| 0 | 2 | 10 | 12 | 0.75 |
| 1 | 8 | 4 | 12 | 0.45 |
| 2a | 5 | 0 | 5 | 0.61 |
| 2b | 5 | 4 | 9 | 0.58 |
| 2c | 2 | 2 | 4 | 0.57 |
| 3a | 5 | 1 | 6 | 0.53 |
| 3b | 8 | 11 | 19 | 0.66 |
| Positive Margins | 0 | 0 | 0 | 1 |
| Skin involvement | 19 | 18 | 37 | 0.98 |
| RMT involvement | 5 | 3 | 8 | 0.8 |
| Facial oedema Above zygoma | 0 | 2 | 2 | 0.046 |
Table 1. The baseline data of the two groups.
Median OS for Non-NACT group is 12 months (95% CI 10.20-13.79) and for NACT Group is 9 months (95% CI 7.29-10.2). It is not significant in Log Rank (Mantel-Cox) (p-value:0.226) but significant in Breslow (Generalized Wilcoxon)(p-value:0.31).
RFS between NACT and non-NACT group
We compared the Recurrence-Free Survival (RFS) between NACT and non-NACT group. Kaplan–Meier analysis showed that the patients in the non-NACT group had a statistically better RFS median 11 months with 95% CI 9.17-12.82) than the patients in the NACT group (median 8 months at 95% CI 6.42-9.57). Their survival curves are shown in Figure 1.
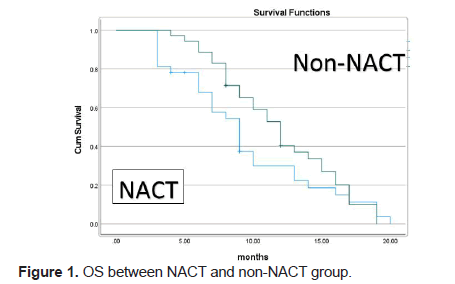
Figure 1: OS between NACT and non-NACT group.
NACT response
12 out of 32 (37.5%) patients who received NACT were good responders including two patients (6.2%) had a Complete Response (CR) and 20 patients (62.5%) had a PR. Kaplan–Meier analysis showed no statistical difference between two groups (p-value: 0.159) with no difference in hazard function, which remain 1.00 at 1-yr. The survival curves are shown in Figure 2.
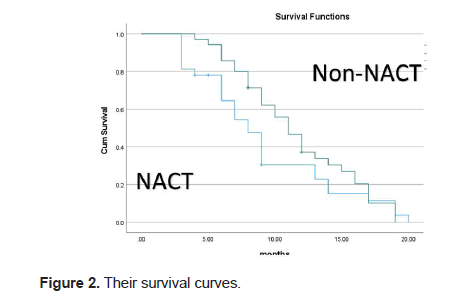
Figure 2: Their survival curves
Influence of NACT on the following surgery
Among 32 patients received NACT, all patients (100%) had radical surgery with flap reconstruction. A choice of conservative or radical surgery is not merely determined by the response of NACT. Multiple aspects patient’s clinical manifestations as suggested in Table 1 were also considered, such as primary site, pathology differentiation, T stage, and N stage. We compared the surgical margins between the patients received NACT or not. None of the patients had positive surgical margins. So, no statistical difference between the two types of surgery (P=1.0) (Figure 3).
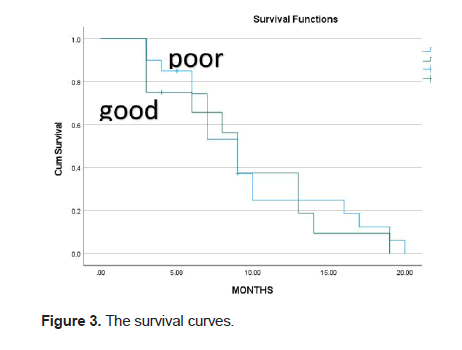
Figure 3: The survival curves
Oral cavity cancers are contributing major portion of overall cancers in both sexes. According recently published data of epidemiology of various cancers in Indian populations suggest that Lip and oral cavity cancers make 10.4% share including the both sexes of all ages. In males, it contributes 16.1% of all cancers and in females; it contributes 4.8% of all cancers [6].
Standard of care in oral cavity cancer is primary surgery followed by adjuvant therapy if required in the form of RT/RT+CT [4].
Neoadjuvant chemotherapy means that administration of chemotherapy before definitive therapy which either may be surgery or RT or RT+CT.
There are various advantages of neoadjuvant therapy which are:
1. Optimal drug delivery: high response rate and transient toxicity
2. Improves nutritional status and performance status
3. Has an established role in organ preservation strategies
4. No compromise of subsequent surgery or RT
5. Is an early systemic treatment for occult disease
6. Overall survival benefit in some study e.g. Wayne state University PF regimen in MACH-NC study
7. New induction chemotherapy regimen e.g. TPF which is more efficacious, less toxic, leads to better quality of life and cost-effective
According to MACH-NC meta-analysis of chemotherapy on HNSCC, survival benefits seen only in concomitant chemotherapy group (HR 0.79, 0.76-0.83 at 95% CI). But most of studies used two drugs regime in induction group and platin only in concomitant group. When compared concomitant to induction group, there are benefits in overall, Event-free survival and local regional failure. Absolute benefit at 5 yrs in concomitant group is 8% (HR 0.81, 0.76-0.88 at 95% CI, p-value <0.0001) as compared to 2% in induction group (HR 0.95, 0.88-1.01 at 95% CI, p-value 0.70). In subgroup analysis, in oral cavity cancers patients with neoadjuvant chemotherapy group suggesting no overall and event-free survival benefit [8].
For respectable advanced oral cavity cancer, a multicentre randomized phase-3 trial by Licitra et al. which including T2 (>3 cms) and T3, N0-2 previously untreated OCSCC giving PF 3 cycles in induction group. 5 yrs overall survival in both arms was 55%. However, mandible resection was performed in 52% of patients in control arm versus 31% in the chemotherapy arm. Postoperative RT was administered in 465 of patients in control arm versus 33% in the chemotherapy arm [9].
After 11.5 yrs of follow-up of above study, Bossi et al. suggested that no difference in OS and DFS, loco-regional and distant metastasis relapse at 10 yrs. But in patients with pathological CR have better survival than no pCR (76.2% vs. 41.3%) [10].
A study by Zhong et al. in which TPF 2 cycles used, no difference in OS and DFS but in subgroup analysis some survival benefit seen in clinical N2 group of patients [11].
So, multiple studies fail to demonstrate improvement in LRC or OS with NACT. But some benefits are 1. Mandibular preservation in tumours without gross invasion of mandible, 2. Reduction in use of post-op RT, 3. Disease control in patients with higher nodal stage, 4. Prognosticate patients based on response.
In unresectable OCSCC as defined by Patil et al. as buccal mucosa cancer with peritumoral oedema reaching up to zygoma, tongue primary reaching up to hyoid bone, extension of tumour in high ITF and extensive skin infiltration impacting achievement of negative margins [12].
In TAX 323 study, patients were randomized to TPF 4 cycles vs. PF 4 cycles both followed by RT. But, in this study, OCSCC patients were 17% of total. OS and PFS benefit seen in 3 drugs regimen vs. 2 drugs. 75% completed 3 drugs regimen [13].
In TAX 324 study, patients were randomized to TPF 3 cycles vs. PF 3 cycles both followed by CTRT and surgery as required. OS and PFS benefit seen in 3 drugs regimen vs. 2 drugs.73% completed 3 drugs regimen [14].
Meta-analysis of 4 main studies for unresectable OCSCC showed no benefit in OS and PFS [15-17].
In borderline resectable OCSCC, 3 retrospective studies done in India. If patients had gone induction chemotherapy followed by surgery had better OS as compared to who had not gone to surgery after induction chemotherapy [12].
NACT which containing 3 drugs regimens have gain popularity recently in locally advanced OCSCC. In our study, it doesn’t made difference in margin positivity after surgery, difference in survival between good and poor responders. According to site, it makes some difference in buccal mucosa cancer with ill-defined oedema at cheek skin. In Non-NACT group, 3 months survival benefit in OS and RFS, this is statistically significant. So, if OCSCC is resectable than resect primarily. If unresectable than we have to determine if it will resectable after NACT than role of NACT is there. But, if not possible after it than patient is candidate for CTRT.
Citation: Patel D et al. Role of Neoadjuvant Chemotherapy in Oral Cavity SCC: A Surgical Oncologists Experience at Tertiary Care Institute. Eur J Clin Oncol, 2021, 3(1), 001-004.
Received: 25-Dec-2020 Published: 15-Jan-2021, DOI: 10.35248/ejco.21.3.004
Copyright: © 2021 Patel D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.