Research Article - (2022) Volume 11, Issue 3
Functional vitamin B12 markers were compared with serum vitamin B12 levels in 350 individuals, to see if there was a correlation between markers of vitamin B12 deficiency, and the actual levels of vitamin B12 in serum. No correlation was found between normal to elevated levels of vitamin B12 and urinary methyl malonic acid, or vitamin B12 deficiency associated neurotransmitter metabolites. Hence, whilst serum vitamin B12 levels were normal or elevated these individuals had Paradoxical B12 deficiency. Vitamin B12 deficiency markers were, however, correlated with markers of vitamin B2 deficiency, suggesting that the vitamin B2 deficiency had led to elevated levels of vitamin B12, which was largely inactive. Given the role in energy metabolism, correct diagnosis of vitamin B12 deficiency is essential. The mechanism is discussed.
Paradoxical vitamin B12 • Organic acids test • Vitamin B2 • Creatine
The current dogma on vitamin B12 deficiency implies a direct link between levels of serum vitamin B12, and sufficiency. Hence as vitamin B12 levels reduce, markers such as MMA and homocysteine increase in an inverse relationship to serum B12 levels [1-4]. A consequence of this "Dogma" is that if a person does not have vitamin B12 levels lower than a highly variable amount, dependent upon study (148 pmol/L, 179 pg/ml, 230 pmol/L), they cannot be B12 deficient [1,5,6]. Hence vitamin B12 deficiency would be mainly due to poor diet, or poor absorption or lack of Intrinsic Factor [7]. Many people, however, experience symptoms of vitamin B12 deficiency yet their serum levels of vitamin B12 may be normal or much higher than normal. Subsequent examination of biochemical markers such as MMA or homocysteine may show that these markers are moderate to highly elevate. As such the symptoms and biochemical markers are indicative of vitamin B12 deficiency, yet the serum vitamin B12 levels are paradoxically high. Such persons are deemed to have "Paradoxical Vitamin B12 Deficiency". Generally, however, the reason(s) for "Paradoxical B12 deficiency" are not known, even despite its association with a greater increase in "all-cause mortality", chronic viral liver disease, Anorexia Nervosa, and death from COVID-19 [8-10].
It has been known for over 40 years that measurements for serum vitamin B12 levels can be greatly distorted by the presence of non-functional cobalamin analogues, and in many cases measurement of serum B12 levels is not predictive of the levels of functionally active B12 [11-20]. In addition, low intracellular folate levels can often be associated with the presence of inactive analogues of cobalamin in serum [21]. Alternatively, it can be associated with inflammatory conditions such as Rheumatoid arthritis due to the overproduction of the B12 binding proteins Tran’s cobalamin and haptocorin, Cystic fibrosis, and patients treated with nitrous oxide poisoning [22-25].
More recently elevated B12 levels have been associated with a poorer prognosis following treatment for cancer [26-29]. The higher the B12, the poorer the prognosis. We have not been able to find any study where the authors have "looked at" metabolic markers that one would ascribe to functional B2 deficiency, and compared it to the elevated B12 levels in these cancer studies. Studies on gastric cancer have suggested that the cancers themselves over-produce haptocorrin (R Binder), and hence elevated serum Hc-B12 may be indicative of prognosis [30-34]. Elevations of serum B12 have also been found in cats with neoplasms [35]. Extreme levels of Hc-B12 (>18,000 pg/m B12) have been found in some metastatic cancers [36].
Despite the almost completely non-predictive nature of serum vitamin B12 levels it is still the measurement of choice for vitamin B12 sufficiency. Its utility is arguably restricted to determining conditions such as dietary insufficiency, poor absorption or Pernicious Anaemia, where serum levels are routinely low, and as such many physicians miss functional vitamin B12 deficiency in patients.
In previous studies we have shown that functional vitamin B12 deficiency is often associated with functional vitamin B2 deficiency. In the current study, we have compared the levels of serum vitamin B12, with various biochemical markers associated with vitamin B12 deficiency, and have compared the later markers with markers of functional vitamin B2 deficiency.
Study sample
A retrospective analysis was performed upon data submitted to us for interpretation from a cohort of 350 children and adults who had submitted their Organic Acids Test data (Great Plains Laboratories, Lenexa, KS, USA) for analysis and who had also supplied their serum vitamin B12 levels. The Cohort, had normal to elevated levels of serum vitamin B12, however, had many symptoms normally associated with vitamin B12 deficiency, including fatigue, depression, anxiety, lack of motivation, poor memory, fuzzy thinking, symptoms of peripheral neuropathy, and difficulty sleeping. In the analysis markers that have previously been shown to be elevated in vitamin B12 deficiency, were analysed and plotted against serum vitamin B12 and methylmalonic acid (MMA) a standard marker of vitamin B12 deficiency. Subjects were from a range of countries including USA, Canada, United Kingdom, Ireland, Germany, Spain, France, Italy, Bulgaria, India, Sweden, Bulgaria, Serbia, Dubai, Croatia and Australia. Age distribution of the 350 individuals was 1-5 (30); 6-10 (30); 11-15 (40); 16-20 (19); >21 (216). Data was collected over a period of 5 years. No selection was made in the acceptance of data, with no data being rejected. Individual data is plotted as Scattergrams (see Graphs 1 to 7). Data is represented as mmol/mol creatine for NT.
Absolute vitamin B12 levels in serum were compared to Methyl Malonic Acid (MMA) levels measured in urinary Organic Acids (OAT). Representation of the data in a scatter gram (Figure 1) showed that there was no obvious relationship between levels of vitamin B12 in serum, and the standard marker of vitamin B12 deficiency, MMA (Methyl Malonic Acid). This is in contrast to the comparison of absolute vitamin B12 deficiency in serum, where MMA starts to increase as vitamin B12 drops below 221 pmol/L ( Figure 1) [6,37].
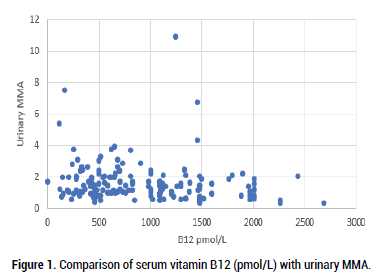
Figure 1: Comparison of serum vitamin B12 (pmol/L) with urinary MMA.
Previous studies by us have shown that deficiency of active vitamin B2 is correlated with the vitamin B12 deficiency markers HVA, VMA, QA, KA and 5HIAA. The level of MMA was compared to the vitamin B2 deficiency marker, glutaric acid (Figure 2), as too were the markers, HVA, VMA, QA, KA and 5HIAA (Figures 3-6).
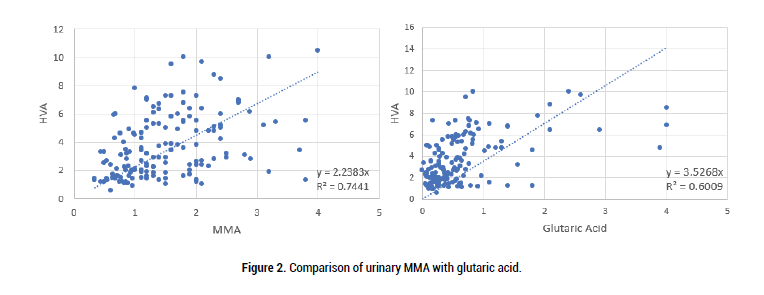
Figure 2: Comparison of urinary MMA with glutaric acid.
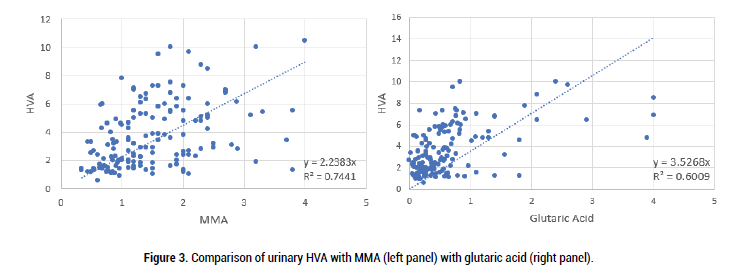
Figure 3: Comparison of urinary HVA with MMA (left panel) with glutaric acid (right panel).
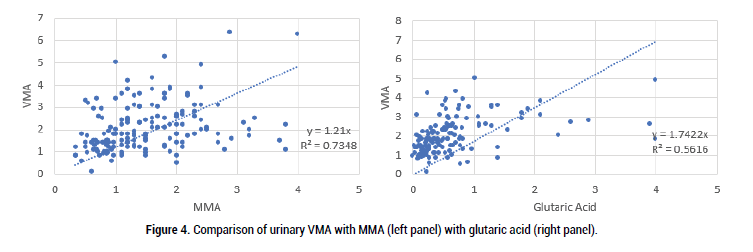
Figure 4: Comparison of urinary VMA with MMA (left panel) with glutaric acid (right panel).
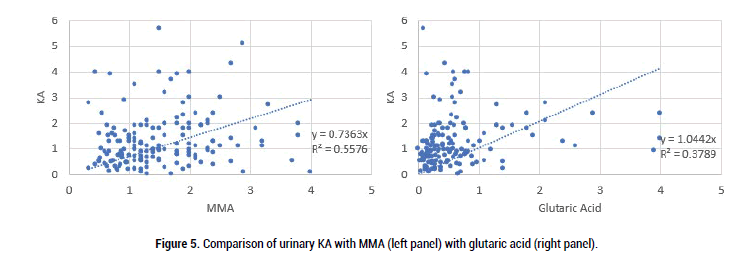
Figure 5: Comparison of urinary KA with MMA (left panel) with glutaric acid (right panel).
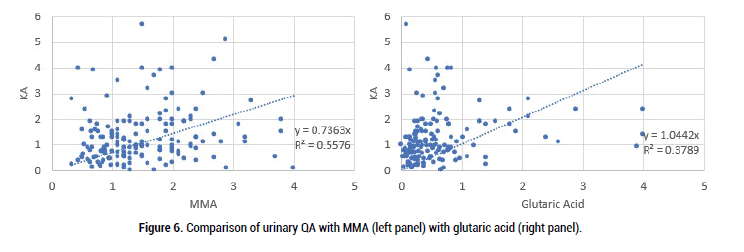
Figure 6: Comparison of urinary QA with MMA (left panel) with glutaric acid (right panel).
As can be seen there was good correlation between both MMA and glutaric acid with VMA, HVA and QA, however, the comparison was less strong for KA. This presumably is because in order to make the metabolite KA, there is a Pyridoxal-Phosphate-dependent enzyme (kynurenine-aminotransferase) step. Formation of the active form of vitamin B6, pyridoxal phosphate (P5P), requires FMN, hence in low Iodine, or Selenium the amount of FMN would be reduced, as too P5P, and so too the amount of KA (Figure 7).
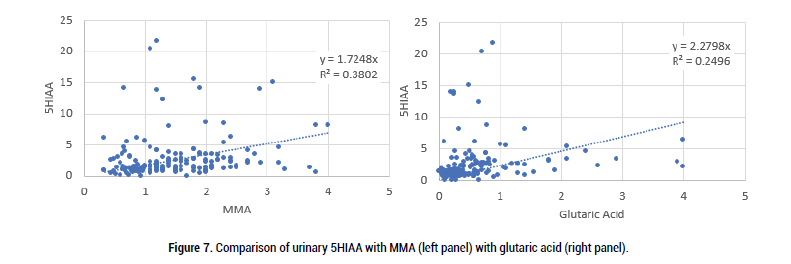
Figure 7: Comparison of urinary 5HIAA with MMA (left panel) with glutaric acid (right panel).
Formation of 5HIAA was less well correlated with either MMA or glutaric acid, however, it is interesting to note that 5HIAA levels tended to increase when glutaric acid levels were lower, a situation that occurs when functional B2 activity is reduced and so presumably the activity of the FAD-dependent enzyme, monoamine oxidase, which is responsible for the inactivation of serotonin to 5HIAA, would be higher. In extreme B2 deficiency, MAO would have reduced activity and so serotonin break-down would be reduced.
In a previous study we compared compared markers of functional vitamin B2 deficiency with those of functional vitamin B12 deficiency, and found a correlation supporting the hypothesis that lack of functional vitamin B2 eventually causes a lack of functional vitamin B12. In this study we have attempted to see if levels of serum vitamin B12 are predictive of functional vitamin B12 levels, and found that they are not at all predictive of functional B12, however, there was a strong correlation between MMA, a marker for Adenosyl B12 deficiency and VMA/HVA/QA (markers of methyl B12 deficiency) and MMA and glutaric acid (a marker of functional B2 deficiency).
The data supports the hypothesis that in functional B2 deficiency, the activity of the two vitamin B12 house-keeper enzymes methylenetetrahydrofolatereductase (MTHFR-FAD/NAD dependent enzyme) and methionine synthase reductase (MTRR–FMN/FAD/NAD dependent enzyme), is greatly reduced and so the repeated cycling of methylCo(III)B12 Co(I)B12 is greatly compromised with the result that Co(I)B12 is rapidly oxidized to inactive Co(II)B12, which cannot be rescued by MTRR in the reaction Co(II)B12+SAM ≥ MethylCo(III)B12+SAH. This inactive cobalamin is then secreted from the cell and accumulates in serum, thereby raising the apparent level of vitamin B12 in serum and yet the B12 is inactive. Subjects with these high levels of inactive B12, therefore have “Paradoxical vitamin B12 deficiency”. This functional B12 deficiency cannot be resolved by additional vitamin B12 until the functional vitamin B2 deficiency is resolved, and the activities of MTHFR and MTRR are restored. The major cause of Paradoxical Vitamin B12 Deficiency appears to lack of functional vitamin B2, which may occur due to overt vitamin B2 deficiency in a person's diet, Hypothyroidism [38], or due to lack of adequate intake of Iodine, Selenium and/or Molybdenum, which in turn leads to insufficient production of the two active forms of vitamin B2, namely FMN and FAD. FMN and FAD both have critical roles in cycling and maintenance of activity of vitamin B12, particularly methyl B12.
Methyl-Co (III) B12 has a major role in the body in the removal of homocysteine, and in the regeneration of methionine in the methylation cycling using the enzyme methionine synthase reductase (MTR).
Functional Methyl-Co (III) B12 is essential for the formation of creatine, the cytoplasmic energy transfer shuttle; hence lack of functional Methyl-Co (III) B12 has important implications for organs with high energy demand,such as the heart, muscles and nervous system. Potentially this explains the observation that “Paradoxical B12 deficiency” is common in conditions such as Chronic Fatigue Syndrome (CFS) and is almost universal in Autism Spectrum Disorder, and in dementia.
Dietary deficiency of Iodine, Selenium and/or Molybdenum can lead to an inability to activate dietary or supplemental vitamin B2. This in turn leads to an inability to maintain the functional activity of vitamin B12. Prolonged lack of active vitamin B2 eventually leads to an accumulation of inactive vitamin B12 in serum, with a gradual increase in the absolute amount of serum B12. Thus, elevated vitamin B12 is found in serum, yet the B12 is largely inactive, leading to a condition of Paradoxical vitamin B12 deficiency.
We declare that there are no potential conflicts of interest.
Research did not involve human participants and/or animals.
No Informed consent is required, all data is “blinded” and as such is anonymous
Data analysis was carried out under the Australian National Health and Medical Research Council guidelines (NHMRC). Under these guidelines, all data was deidentified and steps were taken to ensure the anonymity and confidentiality of the data. Deidentification has consisted of absolute anonymity and confidentiality of the data, such that no specifics such as gender, ethnicity, Country of Origin, etc. are associated with any data point in the study.
As such per the NHMRC guidelines:
• The research does not carry any risk to the participants
• The benefits of the research are many and will be of considerable benefit to any past, current or future participants, and as such represent no harm.
• The data is from over 600 participants collected over 6 years and as such it would be impracticable to obtain consent from the participants. Further the participants had been notified at the time of analysis that data presented for analysis might potentially be used in research–but would be totally de-identified (which it has been).
• There is no known reason why any of the participants would not have consented if they had been asked
• Given the total de-identification of the data, there is absolute protection of their privacy
• Data is only housed in one location and has only been assessed by one person, and as such the confidentiality of the data can be assured.
• No financial benefits from the data are anticipated, rather the data will be used to help prevent and treat those to whom the data applies.
• The waiver is not prohibited by State, Federal or International Law.
Acknowledgements go to all of the individuals who have contributed their data to the preparation of this manuscript in the hope that the manuscript will be of benefit to those who have previously been deemed not to be vitamin B12 deficient, and as a guide to clinicians in their assessment of vitamin B12 deficiency.
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
Citation: Russell-Jones, G. Paradoxical Vitamin B12 Deficiency: Normal to Elevated Serum B12, With Metabolic Vitamin B12 Deficiency. J Biol Today's World, 2022, 11(3), 001-004
Received: 07-Apr-2022, Manuscript No. JBTW-22-59856; Editor assigned: 11-Apr-2022, Pre QC No. JBTW-22-59856(PQ); Reviewed: 25-Apr-2022, QC No. JBTW-22-59856; Revised: 29-Apr-2022, Manuscript No. JBTW-22-59856(R); Published: 09-May-2022, DOI: 10.35248/2322-3308.11.3.003
Copyright: © 2022 Russell-Jones G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.