Research - (2020) Volume 9, Issue 1
Biofilm; Antibiofilm; Streptomyces; Crude extract
In nature bacteria prefer to live in communities with other bacteria in order to compete with the resources and space. Many of the bacteria have the ability to sense the environment around them and behave in a way that they could resist in different environmental niches. [1] One of the ability of most of the bacteria is to form biofilms. Generally, bacteria prefer to live in communities in biofilms as they confer many advantages by adapting such lifestyle. A biofilm is an aggregation of micro-organisms, in which the bacterial cells grow attached to a surface and are embedded in a self-produced extra polymeric substance. It is also known as slime layer, which covers the bacteria in such a way that it acquires resistant to not only antimicrobial agents but also overcome the host immune defense mechanism. Development of biofilms results in structural and phenotypic changes such as colonization of host tissues, expression or enhancement of virulence traits, protection from stress, sequestering of nutrients, enhanced cell to cell communications and survival in harsh conditions [2].
Biofilms can be formed on biotic and abiotic surfaces. They can be formed on, household surfaces in bath and kitchen, in toilet, sinks and pipelines. From medical point of view, they can be formed on implants and medical devices such as mechanical heart valves, catheters, pacemakers, surgical pins, prosthetic joints. Also development of biofilms can occur on contact lenses, on tooth surface as well as on various tissue surfaces. [3]
Biofilm may consist of a single species or multi species community. Single species biofilms are mostly involved in variety of chronic infections. Therefore, study of single species biofilms is of great interest in research [4].
Biofilm formation occurs in a series of steps. The first step involves initial attachment of planktonic bacteria to a solid surface, followed by production of extra polymeric substance, resulting in proliferation of attached cells and accumulation of more cells forming multilayer cell clusters which are called as micro colonies. This forms the early stage of biofilm architecture. The micro colonies further proliferate and form a mature biofilm. In the Last step the single cells disperse from the matrix [4,5]. The matrix covers the surface and allows the cells to resist against different environmental conditions. The matrix acts as a stabilizing scaffold for the three dimensional biofilm Nutrients get trapped in the matrix for metabolic utilizations by the resident bacteria and with the aid of hydrophilic polysaccharides, water is efficiently retained through H-bond interactions. Enzymes secreted by the bacteria modify EPS composition in response to changes in nutrient availability, resulting in changes in biofilm architecture to the specific environment. The structural components of the matrix are responsible for a highly hydrated, robust structure with high tensile strength that keeps bacteria in close proximity, enabling intimate cell-to-cell interactions and DNA exchange, and also protects the biomass from desiccation, predation, oxidizing molecules, radiation, and antimicrobials [6]. The resistant nature of biofilms can also be attributed to the presence of environmental gradients within the biomass, which give rise to community with subpopulations of bacteria showing differential gene expression in response to local nutrient and oxygen availability [7].
The composition of extra cellular matrix varies among different bacterial species. It consists of exopolysaccharides, which is an essential component of almost all bacteria that provide a matrix for initial attachment of cells. Beta-1,6- linked Nacetyl-Dglucosamine is the most common component of many bacterial biofilm [8]. Besides these proteins, Pilli, flagella and other adhesive fibres and extra cellular DNA are also present in the biofilm.
Biofilms show negative impact on industry, marine transportation and medicine. The increasing rate of antibiotic resistance, has become a threat to public health. They are involved in variety of human infections such as endocarditis, osteomyelitis, chronic otitis media, gastrointestinal ulcers, urinary tract infection, chronic lung infections like cystic fibrosis patients and pariodontitis. The infection which is most widely reported is catheter associated urinary tract infection. Many of the chronic infections like diabetic, ulcers result from bacteria growing in biofilms.
Both gram positive and gram negative bacteria are capable of forming biofilms.
Klebsiella pneumoniae is one of the most important pathogen causing infections such as pneumonia, sepsis and urinary tract infection [9]. Besides K. pneumoniae is the major cause of infections in catheterized patients and therefore it is considered as top eighth nosocomial pathogen [10]. It is characterized by the virulence factors such as capsule, type 1 and type 3 pili, KPF-28 fimbriae and lipopolysaccharides. Among these capsule allows it is overcome phagocytosis and cause infection [10].
Staphylococcus aureus is an opportunistic pathogen that forms biofilms on medical devices and contact lenses. This organism is involved in nosocomial and chronic biofilm-associated infections. If forms biofilm by initial attachment of surface proteins to host proteins, which is followed by exopolysaccharides expression that allows aggregates of bacterial cells.
Pseudomonas aeruginosa is a pathogen involved in both acute and chronic infections. It can form biofilm on tissues; it is a major problem in burn wounds, chronic wounds, surface growth on implanted biomaterials, and hospital surfaces.
Recently it has been reported that Enterobacter cancerogenus cause infections in adults like wound infections and septicemia. It was also reported that this organism forms biofilm on the neonatal enteral feeding tubes. Therefore, neonatal feeding tubes can be considered as potential source infections in neonates.
Thus, due to the increasing resistance towards antimicrobial agents, research on how bacteria form biofilms and how these biofilms can be inhibited by various nontoxic compounds without affecting the growth of organisms is under focus.
The Actinomycetes are aerobic gram positive bacteria, with a high GC content, able to form branching filaments or hyphae. They resemble fungi in their morphology. They belong to the phylum Actinobacteria, they are found in both terrestrial and aquatic ecosystems, mostly in soil. Around 23,000 biologically active secondary metabolites are known to be produced by micro-organisms of which approximately 45% are produced by actinomycetes. Among Actinomycetes majority of bioactive compounds are produced by Streptomyces species. Streptomyces have therefore become a hotspot for pharmaceutical industry. Members of this group are producers of antimicrobial, antiparasitic, antichlamydial, antioxidants, immunomodulatory, anticancer and antibiofilm agents.
Recently some reports have shown that, actinomycetes produce certain active antibiofilm agents for example, Streptomyces albus have shown to inhibit the biofilm of Vibrio harveyi, and Streptomyces akiyosheinsis inhibits the biofilm of Staphylococcus aureus [1].
Present study aimed to explore the potential of Streptomyces viridochromogenes strain NBRC 3113 and Streptomyces levis strain NRRL B-16370 to produce certain broad spectrum antibiofilm agents [11-18].
Test bacterial isolates
Pseudomonas aeruginosa and Staphylococcus aureus were obtained from hospital. Klebsiella pneumoniae and Enterobacter cancerogenus were isolated from environmental sample. These four organisms were examined for their biofilm forming ability.
Screening bacterial isolates for their biofilm forming ability
Congo red assay (CRA): Congo red agar (Brain heart infusion broth-37 gm, sucrose-5 gm, Congo red dye-0.8 g, and agar-15 g per litre of distilled water) was used for selection of biofilm forming organisms. Congo red agar plates were inoculated with different organisms as Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae and Enterobacter cancerogenus and incubated for 24 hrs 37°C. After incubation colour of colonies were observed. Black colour colonies indicate organisms are biofilm producing.
Biofilm formation on glass surface: Sterile glass test tubes containing 2 ml of nutrient broth were inoculated with each bacterial suspension. Tubes were incubated with for 24 hrs at 37°C. Planktonic bacteria were discarded and tubes were washed with PBS and allowed to dry. Tubes were then stained with 0.3% of crystal violet. After 10 mins excess stain was discarded and tubes were washed, dried. Tubes were observed for their ability to form a biofilm ring onto the surface of glass.
Capsule staining: Capsule staining was performed using standard protocol.
Isolation of actinomycetes from different sources
Samples for Streptomyces cultivation were collected from different sources like from soil, chick slaughter wastes; a swab from hand was taken. These 3 samples were inoculated in each 100 ml flask containing Bennet's broth medium (Yeast extract 1 g/L, Beef extract 1 g/L Casein enzyme hydrolysate 2 g/L, Dextrose 10 g/L pH 7.3). Enrichment of actinomycetes was carried for 5 days in a rotary shaker (150 rpm) at 28°C. Isolation of Streptomyces was then carried out by spread plate method. For this purpose, sterile Bennet's agar Petri plates were prepared. 0.1 ml of enrichment broth was spreader with a sterile spreader on several plates. The plates were incubated for 7 days at 28°C. Plates which showed zone of inhibition around a colony and which appeared chalky white in colour were subculture on new sterile Casein starch agar plates.
Microscopic examination
Microscopic examination of actionomycetes was done using standard slide culture method.
16S rRNA sequence determination
The isolated actinomycetes were sent for genetic identification by 16S rRNA technique to IISER Pune.
Extract preparation
Single colony of each isolated actinomycetes culture were inoculated in 150 ml Bennet's broth medium in 250 ml of conical flask and the strains were allowed to grow for 10 days at 28°C on a rotary shaker at 150 rpm. After centrifugation, the filtrates as well as the supernatants were treated with twice the volume of ethyl acetate separately. Bennet's broth medium served as control for the process and was treated in the similar manner with ethyl acetate and it was used as medium control for the assay. The extract was generated by evaporating the solvent for 48 hrs at room temperature. The dried extracts were dissolved in DMSO (final concentration 3.75% on the cells) and used for the antibiofilm bioassay.
Effect of actinomycetes crude extract on pathogenic biofilms
To study the effect of crude extract, biofilms of pathogenic bacteria were developed. For this bacterial suspensions were made (OD600= 0.05). 100 ml of bacterial suspension was inoculated in 96 well flat bottom polystyrene plates containing 200 ml of nutrient broth. The plate was incubated for 48 hrs at 37°C. Plantonic bacteria (free cells) were discarded by inverting the plate.100 ml of crude extract was added to the existing biofilms and plate was incubated for 24 hours at 37°C.Plate was washed with sterile phosphate buffered saline twice, and was heat fixed at 65°C for 1 hr. 100 micro litre of 0.3% crystal violet was added to each well. After 5 mins plate was washed with distilled water and dried. 200 ml of acetic acid was added to each well to dissolve the content in the well and OD 492 readings were compared with the control. Biofilms which were not treated with extract served as controls with Bennett’s medium as extract control.
Determination of biofilm inhibitory concentration (BIC 50)
Biofilms were formed in 96 well microtitre plates. The biofilm was treated with 80, 160, 240, 320, 400 μg/ml concentration of crude extract for 24 hrs 37°C. After treatment biofilm was washed with PBS and stained with 0.3% crystal violet for 5 mins. After staining plate was washed 2-3 times with water and OD was measured at 492 nm. Depending on OD biofilm inhibitory concentration was determined.
Growth measurement
Effect of actinomycetes crude extraction the growth of different biofilm forming bacteria was studied by growth curve analysis. The growth curve of these bacteria was determined upto 24 hrs. The crude extract at 50% BIC50 and highest tested concentration was added to a conical flask containing bacteria of an OD 0.1 at 600 nm. These flasks were incubated at 37°C, 200 rpm. Nutrient broth without bacteria serves as a negative control. Growth medium with crude extract and bacteria serve as the control. The reading was observed continuously up to 24 hrs at 2 hrs of interval. Experiment was carried out with four independent cultures.
Physical and chemical treatment to the extract
To know the physical and chemical characters of the extract, they were subjected to heat and enzymatic treatment. The extracts were heated at 70°C and 100°C for 30 mins and cooled on ice. For enzymatic treatment, the extracts were treated with proteinase k (20 mg/ml).This mixer was incubated for 1 hour at 37°C. Extracts without enzyme served as control. Both the treated extracts were examined for their antibiofilm activity by crystal violet assay.
Cell surface hydrophobicity assay [Microbial adhesion to hydrocarbons (MATH)]
In 2.5 ml of phosphate buffer saline bacterial cells from different cultures were suspended in different tubes. 0.5 ml of xylene was added to the tubes. This mixture was incubated at 44°C fir 10 mins. After this samples were vortex for 60 sec and were further incubated for 1 hr. The hydrocarbon and aqueous layer of the mixture were allowed to separate. Optical density of the aqueous phase was measured at 600 nm. The decrease in the OD of aqueous layer was the measure of cell surface hydrophobicity.
Selection of biofilm forming organisms
Congo red assay: Organisms showed black colour colonies on Congo red agar plate which confirmed that they are the biofilm producers (Figures 1).
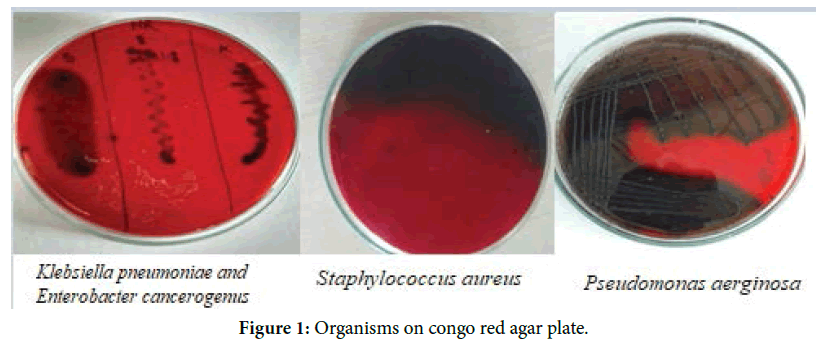
Figure 1. Organisms on congo red agar plate.
Tube assay: Biofilm formation on glass surface by test bacterial isolates (Figure 2).

Figure 2. Biofilm formation on glass surface by test bacterial isolates.
Capsule staining: 4 different organisms were shown (Figure 3).
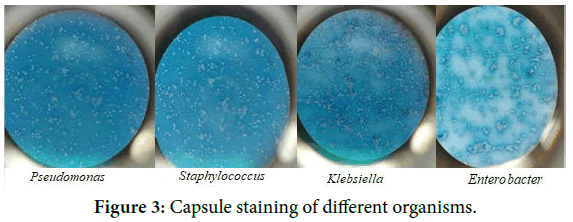
Figure 3. Capsule staining of different organisms.
Isolation of actinomycetes
The characters of strains are tabulated in Table 1 and shown in Figure 4.
| Characters | Streptomyces viridochromogenes strain NBRC 3113 | Streptomyces levis strain NRRL B-16370 strain NRRL B-16370 |
|---|---|---|
| Color | Bluish green | Off white |
| Size | 1 mm | O.5 mm |
| Elevation | Flat | Flat |
| Margin | Irregular | Irregular |
| Consistency | Dry | Dry |
| Opacity | Opaque | Opaque |
| Gram staining | Gram positive | Gram positive |
Table 1: Characteristics of Streptomyces viridochromogenes and Streptomyces levis.
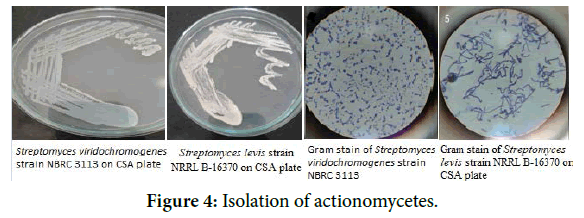
Figure 4. Isolation of actionomycetes.
Microscopy examination
The isolates were examined using slide culture technique under 40X and 100X. The microscopic examination showed filamentous aerial hyphae (Figure 5). The RNA Sequence determination has been shown in Figures 6 and 7. The Effect of crude extracts on bacterial biofilms tested at highest concentration is shown in Figure 8.
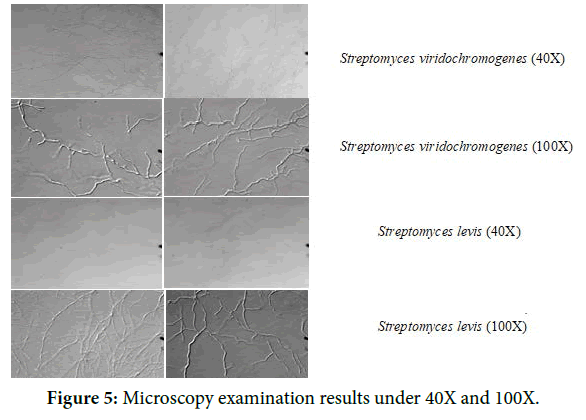
Figure 5. Microscopy examination results under 40X and 100X.

Figure 6. 16S rRNA sequence of Streptomyces viridochromogenes strain NBRC 3113 strain.

Figure 7. 16S rRNA sequence of Streptomyces levis strain NRRL B-16370 strain.
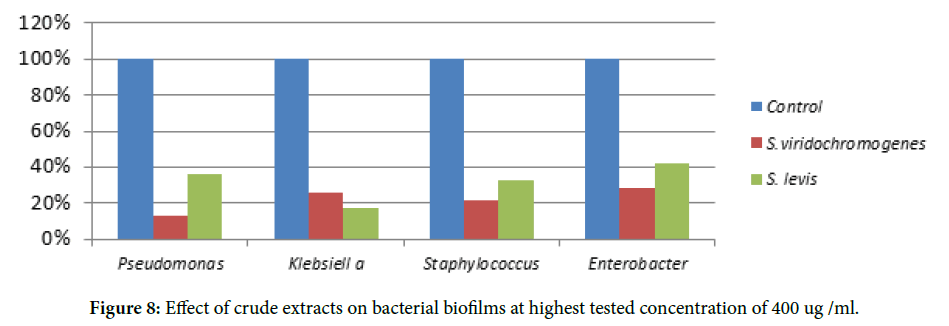
Figure 8. Effect of crude extracts on bacterial biofilms at highest tested concentration of 400 ug /ml.
The biofilm inhibitory concentration has been determined in Figures 9 and 10.
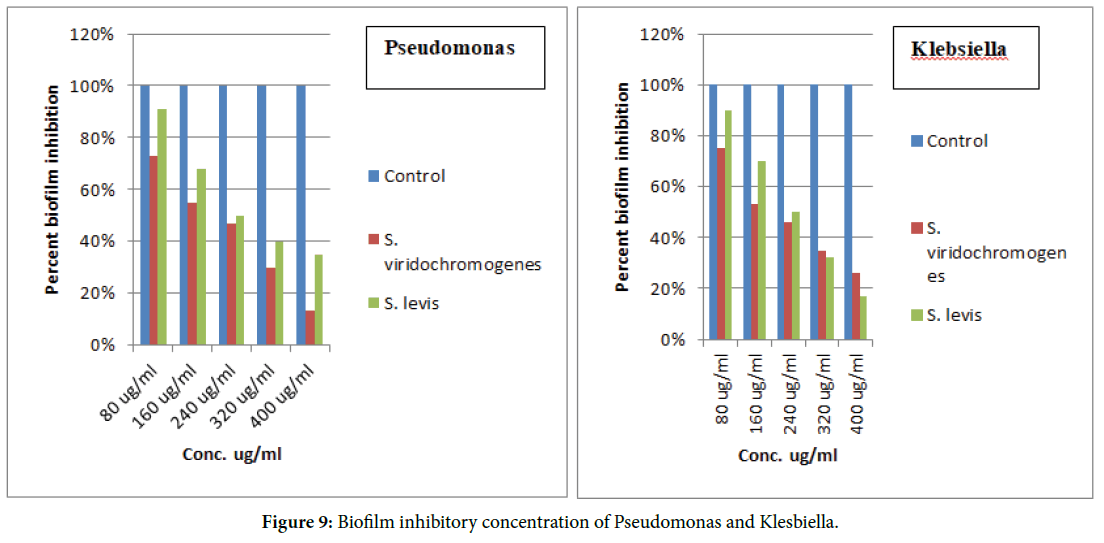
Figure 9. Biofilm inhibitory concentration of Pseudomonas and Klesbiella.
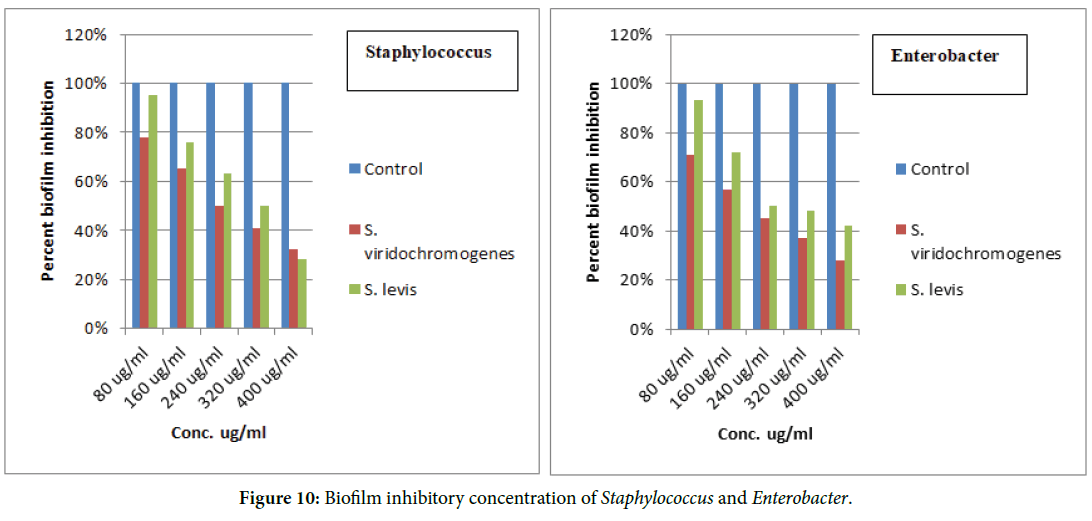
Figure 10. Biofilm inhibitory concentration of Staphylococcus and Enterobacter.
Physical and chemical characterization of active compound in the extracts
Physical treatment (Heat): Streptomyces viridochromogenes strain NBRC 3113 is heat resistant and Streptomyces levis strain NRRL B-16370 is heat labile.
Chemical treatment (proteinase K): Both extracts are non proteinaceous.
The Growth Curve of the organisms is shown in Figure 11.
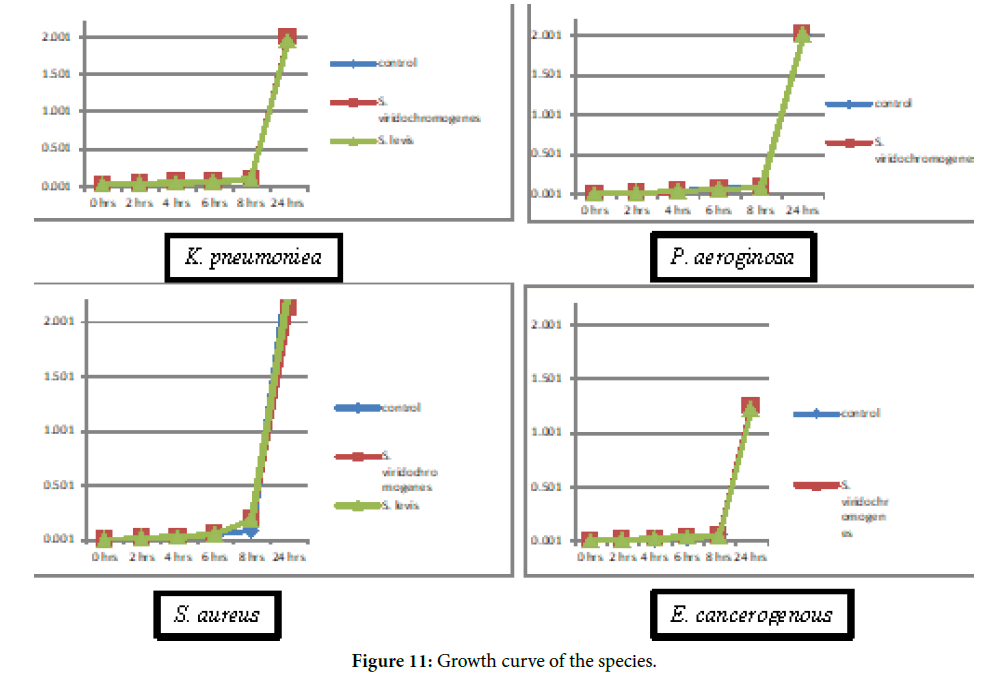
Figure 11. Growth curve of the species.
Hydrophobicity
Surface hydrophobicity plays an important role in most of the biofilm forming organisms. It helps to adhere to the hydrophobic surfaces. For this reason, cell surface hydrophobicity assay was performed to determine the mechanism of inhibitory effect of the extracts. The assay results showed that in presence of extracts the cells become less hydrophobic (Figure 12).
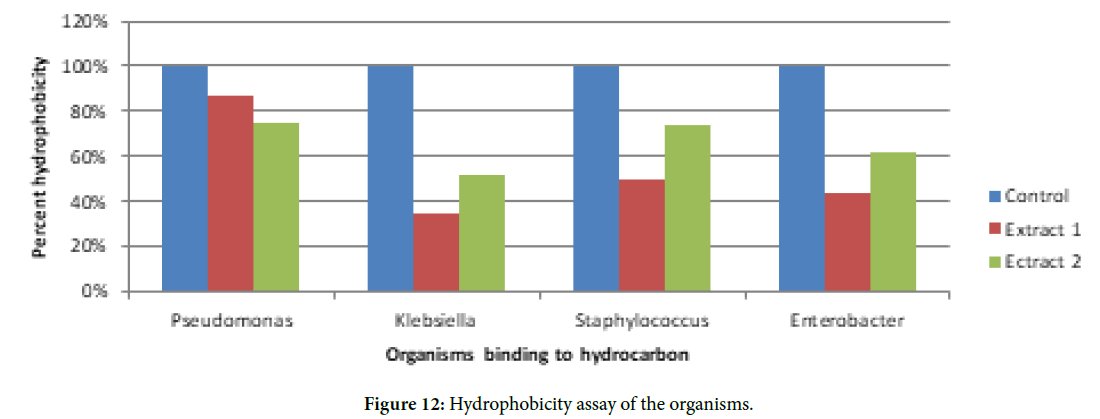
Figure 12. Hydrophobicity assay of the organisms.
Since biofilm formation nowadays has become a major problem, the need for developing new antibiofilm agents is under focus. Previous studies reported that marine Streptomyces extracts inhibit biofilm formation of Staphylococcus aureus but it was not effective on Pseudomonas species [1]. The aim of the present work was to isolate Streptomyces from different sources like chicken slaughter waste and screen for its antibiofilm activity. These Broad spectrum Streptomyces extracts not only inhibited biofilm of Staphylococcus but of Pseudomonas Klebsiella and Enterobacter spp. Previous studies presented antibiofilm activity of various Streptomyces species like Streptomyces sp. MCO25 and Streptomyces sp SBT343 extracts inhibit Staphylococcus aureus biofilms [1,15]. Current study presented the potentia l of Streptomyces viridochromogenes strain NBRC 3113 and Streptomyces levis strain NRRL B-16370 extracts to inhibit biofilms of bacteria for the very first time.
From the present study it can be noted that, apart from marine environment, there are large number of bioactive compounds present in different sources which are still unexplored. The two extracts derived from Streptomyces viridochromogenes strain NBRC 3113 and Streptomyces levis strain NRRL B-16370 showed antibiofilm activity against various biofilm forming organisms. The extracts are non-proteinceous in nature. Streptomyces viridochromogenes strain NBRC 3113 extract is heat resistant and Streptomyces levis strain NRRL B-16370 extract is heat labile. In the presence of extract, the bacteria showed decrease in their adherence property to hydrocarbons. The extracts are dose dependent and non-toxic. Further purification and characterization of the active component in the extracts would reveal the potential role in inhibition of biofilm and the component can be used to develop an active antibiofilm agent.
Purification of the active component from the crude extracts and their structural identification.
Identifying the mechanism by which inhibition is occurring.
Study of gene expression, involved in biofilm formation, in presence of crude extract.
Testing the same extracts against other biofilm forming organisms to prove their broad spectrum antibiofilm capacity.
We would like to thank the department of Microbiology, Abeda Inamdar Senior College, Pune. We would specially like to thank Dr. Jaspal Kaur Oberoi for her valuable guidance and for giving us the opportunity to work and all the teaching staff for their timely encouragement during our entire course.
Received: 03-Jan-2020 Published: 20-Jan-2020, DOI: 10.35248/2322-3308.20.9.211
Copyright: © 2020 Oberoi JK, et al. This is an open access paper distributed under the Creative Commons Attribution License. Journal of Biology and Today's World is published by Lexis Publisher.