Editorial - (2020) Volume 2, Issue 1
Glioblastoma (GBM) is a fatal type of brain cancer or primary glial neoplasm, mostly targeting aged populations. The average survival is only 15 mouths from the date of occurrence. It is often observed, the invasive nature of tumour is the main reason for poor prognosis and strong recurrence of GBM in patients even after prescribed treatments. Despite all type of therapy, it is necessary to understand the various molecular mechanisms and signalling pathways to identify targets for GBM treatment. This compilation is specifically design to discuss about Wnt signalling and Hedgehog-GLI1 pathways which have positive and negative regulation against GBM. The recent research finding associated with different signalling pathways for GBM was also been discussed within this article.
Glioblastoma (GBM), Wnt signalling, Hedgehog-GLI1, Rho/Rhzo kinase (ROCK), β-catenin
Glioblastoma Multiforme or Glioma (GBM) is one of the most devastative glial solid neoplasms [1]. The GMB cell has higher tendency to infiltrate local cells and could produce cerebral necrosis [2]. Due to this reason surgery, wafer technology, radiation therapy, chemotherapy has failed primarily to prevent possible reoccurrence after suitable therapy [3]. In surgical therapy; hemispherectomy could not improve overall survival period of the GMB patients [4]. Therefore, it is become necessary to study various incursion pathways of GBM to understand its proliferative nature. An invasion or incursion pathway of GBM involves interaction within white matter, extra cellular fluid and cancer cells which is superficially regulated by 140 types of genes [5]. Many multiregulated pathways Viz. P13K/Akt, Wnt, Micro RNAs and Sonic hedgehog has shown invasive characteristic of Glioma [6]. The different types of GBM i.e., pro neural (PN), neural, mesenchymal shows differences in asymmetric genetic profile. To understand the actual infiltrative potential of GBM it is prerequisite to understand various invasive pathways and heterogeneity of GMB. However, we live in an era of infancy as far as GMB infiltrative pathways are concerned. The main agenda of this editorial opinion is to highlights recent mechanises and highlight major research finding associated with GBM [7]. From the cross sections of the GBM patient brain it was witnessed that, Glioma implicated secondary structure or Scherer ’ s within brain parenchyma. It also developed a seperate physiological condition within brain with pre-existing complexed blood vessels. These invasive cells further outspread over white matters and subarachnoid space [8]. Surprisingly GBM cells possessed different shape in different conditions; this is could be due to the different pathways of GBM. Immigration of GMB cells to adjacent tissue has resemblance with migration of embryogenic cells [9]. The membrane potential fluctuation and morphological polarization are responsible for migration. It is often witnessed; GBM cells bind with extracellular fluid and elevate it forward. Many growth factors i.e., cell extracellular matrix, chemokines helps in alternating shape, size and volume of GBM cells; which ultimately helps in migration within inter and extracellular spaces. In this migration Metalloproteinases (MMP) helps as a catalyst within extracellular membrane [10]. Therefore, MMP acts as a biomarker for GBM. It was often seen GBM cells has MMP expression higher as compare with the normal cells [11]. Not only extracellular protein, but certain enzyme like cathepsin B, cathepsin D and heparinase helps GBM cells to migrate. It is also witnessed that Rho/Rhzo kinase (ROCK) helps in mesenchymal migration. GBM cells sometimes shows resembles properties like steam cells properties of GBM acting like a potential barrier for penetrating therapeutic agents inside [12]. This glioma initiating cells (GICs) or glioma steam cells (GSCs) can easily be identified using certain cell surface markers CD133 and Nesting. While cell invasion, another protein called Glutamate oscillate Ca2+ intercellular exchange and promote AMPA receptors. As I earlier discussed GMB invasion has resemblance with wingless/Int1 signalling pathway or embryogenesis pathway. Not only in GBM but in breast cancer, colon cancer, and leukaemias this type of wingless/Int1 signalling pathway was palpable. In GMB, Wnt ligands Wnt1, Wnt 2, Wnt3a and Wnt5a are mostly over expressed. Recent research concluded that, Wnt1 causes non-invasive small glial tumour in mice model. One of the most significant and worldwide studied pathway is β-catenin dependent pathway. This pathway began when Wnt ligands binds transmembrane (Figure 1A). While this interaction, Wnt factors and Fz factor interact with coreceptors and low-density lipoprotein (LRP5/6) [13] which forms a complex which disbalanced cytoplasmic scaffolding protein. Furthermore, the complexes translocate itself within nucleus and interact with T-cell factor (TCF) and lymphoid enhancer factor (LEF) to regulate the expression of genes. These target genes include C-Myc, cyclin D1, and MMPs. The C-Myc and cylin D are responsible for proliferation of cancer cells and MMPs helps in increase the potential of MMPs. Wnt factors which are well known to activate the β- catenin are Wnt1, Wnt 3a and Wnt 7a. due to β-catenin, GBM gain invasiveness property. In non-canonical dependent PCP pathway (Figure 1B) Wnt signaling at Fz activates a mitogen activated protein kinase called Jun-N-terminal kinase [14]. The upregulation of GBM activated using Wnt2, Wnt4, Wnt5a, Wnt5b, Wnt6 and Wnt1 [15]. Another most significant pathway is Hedgehog Pathway; which is very critical in embryogenesis and in tumour genesis. Three types of ligands such as sonic hedgehog (Shh), Indian hedgehog (ihh), and Desert hedgehog (Dhh) can effectively activate Hedgehog pathway [16]. The receptor responsible to this pathway is called transmembrane receptor patched (PTCH), binding ligand with the receptor rescinds another 7- transmembrane receptor protein called Smoothened (SMO) [17]. The pathway is depicted in Figure 2. The SMO upregulates several genes like GLI1 PTCH1, cycin D and VEGF. These pathways are activated by tyrosine kinase receptors like EGFR and PDEFR. tGLI has also been shown to upregulate the expression of VEGF-A and heparinase. This type of conditions leads to increase vascularity of GBM as compare to normal GLI variants. To target GBM, it is necessary to target GLI by some good therapeutic agents. Recently Liang L et al. (2019) research suggesting that Glioma steam cells can produces GBM through TLR9/ NEAT1/STAT3 pathway when subject is infected with Adenovirus [18]. Another research conducted by Yang H et al. (2020) highlighting the fact that, Temozolomide-induced Glioblastoma cell death is due to the SIAH1 – HIPK2 – p53ser46 damage response pathway [19]. The research also suggesting that the ATM/ATR target SIAH1 together with HIPK2 plays a proapoptotic role in glioma cells exhibiting p53wt status. As per Rajesh Nanta et al. (2019) research, the suppression of PI3K/Akt/mTOR pathways could initiate tumorigenic potential of Glioblastoma cells [20].
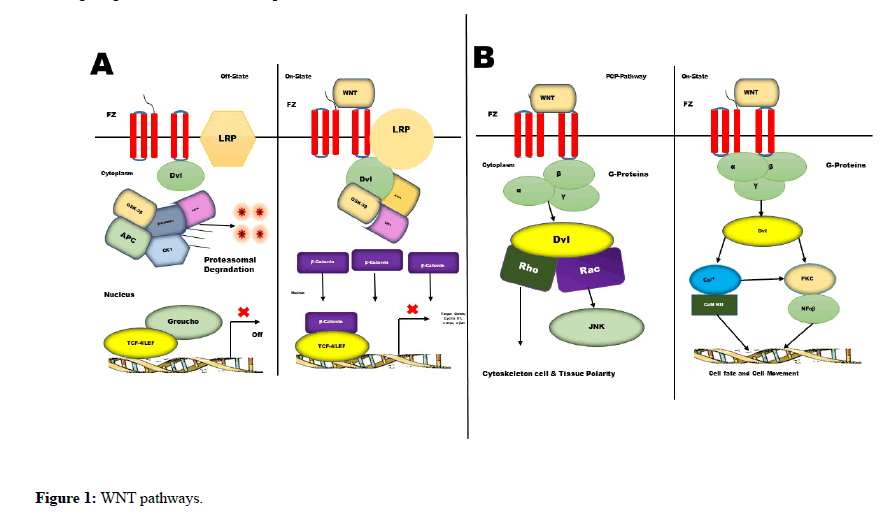
Figure 1: WNT pathways.
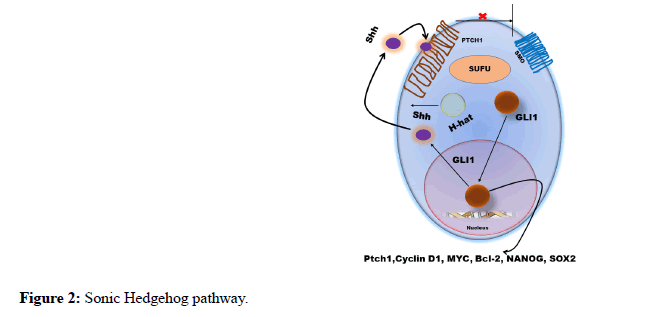
Figure 2: Sonic Hedgehog pathway.
In recent years, many researches are going on to identify the pathways and molecular mechanisms. But we are still in infancy to understand GBM. Hopefully, in upcoming days this terrible disease can be cured by proper understanding of pathways and its associated mechanisms.
Received: 18-Apr-2020 Published: 08-May-2020
Copyright: © 2020 Bhattacharya S, This is an open access paper distributed under the Creative Commons Attribution License. European Journal of Clinical Oncology is published by Lexis Publisher.