Research Article - (2020) Volume 9, Issue 2
Achievement of chemotherapeutic drugs to resistant cancers such as colorectal cancer has been a point of focus in research settings both in clinical and basic science. Identification of potential biomarkers which can help to counteract the effect of resistant drugs by enhance the activity of chemotherapeutic agents is the best option for controlling the treatment of this second leading morbidity metastatic tumour type. RPN II as an identified bio-marker has been showing a positive results as good biomarker for colorectal and other malignant tumours. This review focuses on describing role and mechanism through which RPN II are involved in colorectal cancers basing on its expression and interaction with proteins affecting cell proliferation, growth, tumorogenicity and treatment especially on resistant chemotherapeutic cancer cells and drugs.
Chemotherapy, Colorectal cancer, EGFR, Ribophorin II.
Colorectal cancer (CRC) has been ranked as among of the most common gastrointestinal malignant tumour with more than 1.2 million patients diagnosed and enrolled for treatment each year and leading to more than 600,000 annual deaths worldwide [1,2]. The incidence of CRC has become more raised currently due to both potential external environmental factors such as socio- economic as well as internal factors including unhealthy lifestyle, diseases, and genetic predisposition factors [3-6]. The magnitude of the problem gradually has also seen to increase in Asia which has been associated with the high rate of Aging population compared to the global societies [7,8]. The median age of diagnosed patients lies 60-75 years [9,10]. The growth characteristics of colorectal cancer usually begin as an adenomatous polyp, progressing to an advanced adenoma, then to invasive cancer. Along with the phenotypic progression during the development of colorectal cancer, there are identified associated genomic alterations that drive the multistep process towards invasive disease [11,12]. It has been pointed by various studies the essential characteristics of malignant tumours are excessive proliferation, differentiation failure and apoptosis disorder causing most patients die from tumour metastasis and recurrence [13,14]. These processes of malignancies have been indicated to be controlled by glycoproteins and their associated mediators. Therefore, it is important to explore the mechanisms of tumour growth, metastasis and recurrence in CRC with a focus on essential specific biomarker which will be highly predictive for providing proper diagnostic and therapeutic target for improving patient quality of treatment and life.
Ribophorin II (RPN II) is a major membrane glycoprotein which is embedded in rough endoplasmic reticulum at chromosome 20q12- 13.1 [15]. This glycoprotein component plays glycosylation function affecting protein stability, secretion and cell function, signal and transduction processes [16,17]. Ribophorin II is highly expressed in tumor stem cells and has been identified and used as prognostic marker of several human cancer, including colorectal, breast and pancreatic cancers [18]. Studies revealed RPN II to be a unique integral rough ER membrane glycoprotein involved in translocation and the maintenance of the structural uniqueness of the rough ER [19-21]. RPN II has also been revealed to be useful target toward the regulation of resistance of tumor cells to the chemotherapeutic agents, such as docetaxel and taxane, in both clinical and animal models of breast, ovarian cancers, and esophageal squamous cell carcinoma making it a novel biomarker and promising option towards colorectal cancers [18,22]. Studies shows that RPN II controlling its expression and silencing will confers drug resistance by N-glycosylation, which then stabilizes the transporter P-glycoprotein (P-gp) in the cellular membrane, and regulating anti- apoptotic genes. RPN II expression status in patients with several cancers like breast cancer, Osteosarcoma and oesophageal cancer has also been associated with the effective response to docetaxel thus proposing RPN II as a good candidate predictive marker for chemotherapeutic resistance to drug including docetaxel-based chemotherapy [19]. High expression of RPN II in CRC makes it an interesting point for development and therapeutic targets for CRC treatment. This review focuses to elucidate the information about functional role and mechanism of RPN II in colorectal cancer basically its role and mechanism on chemotherapeutic resistant drugs.
RPN II as a potential biomarker has been highly expressed on both patient samples and colon cancer cell lines tested where its down regulation entailed less development of CRC providing its mechanism through protein glycosylation to the cell and induce regulation of cell function by affecting protein stability, localization and secretion [1,23]. Studies shows CRC like other malignant tumor cells have the characteristics of proliferation, migration and invasion a molecular regulator is of paramount important and studies have indicated the low expression of RPN II suppress the process of cell cycle, restraining cells in the G1- phase, inhibiting cell proliferation and promoting cell death [1,24]. This has lead RPN II to be an area of focus and promising biomarker which associated with CRC metastasis [11].
Inhibition of metastasis in Cancer cell have been identified and used as management option to reduce the rate of cancer progression and its associated effects. Several studies identified several biomarkers and inhibition sites of cancer growth and apoptosis. Studies using siRNA knock down RPN II expression on gastric cancer cells shows remarkable responsiveness of cancer cell to chemotherapy drugs [19]. In various human malignancies, silencing of RPN II it has been associated with increased apoptosis, reduced tumor growth and increased sensitivity of tumor cells to some drugs including docetaxel response shows that siRNA-mediated RPN II knockdown in the AGS cells increased the percentage of apoptotic cells from 5.9% in the siRNA control cells to 8.6% in the RPN II-knockdown cells and additional induction of apoptosis was observed after treatment with cisplatin, which increased the percentage of apoptotic cells between 5.9% in the control siRNA group and 14.4% in the RPN II-knockdown cells [18,25,26]. This study signifies that RPN II silencing and knockdown enhances drug-induced apoptosis as per Cisplatin- induced apoptosis.
Down regulation of Ribophorin II (RPN II), has also efficiently induced apoptosis in docetaxel- resistant human breast cancer cells in the presence of docetaxel, and RPN II silencing repressed tumorigenicity and sensitized the tumours to cisplatin treatment [26-28]. On other side, high expressed RPN II had a significantly lower 5-year survival rate and shows high recurrence rate of gastric cancer cases compared with low RPN II expression in gastric cancer cases. These results therefore indicate that RPN II has also a role to play in CRC as Literature have shown that its expression is associated with worse prognosis in CRC and other tumours when it is silencing or down regulated the mechanism is the same to other cancer site and it is expression is crucial in predicting for tolerance to combination chemotherapy of docetaxel and cisplatin [19,26,29].
Metastasis of cancer cells require a remarkable marker which can help to identify, classify and target cancerous cells for treatment. Several studies have tried to identify a marker through which resistant and non-respondent cancer cells to treatment can be managed and it have been shown that expression of some proteins are necessary and predicts to the chemotherapeutic resistant cancer cells, the prognosis of several cancers has improved over recent years which has resulted to improved surgical techniques such as three-fold lymph nodes to some areas such as Japan though further improvements on identification of biomarker which can be used as indicator for prognosis and assessment of chemotherapeutic responsiveness are required [25,30-32]. Chongyao Bi et al. described the role of N-oligosaccharyltransferase complex (RPN2) in the regulation of squamous cell carcinoma whereby the study shown down regulation of RPN2 is the predictive response to the chemotherapeutic resistance agents in squamous cell carcinoma such as of breast cancer, esophagus cancer and adenocarcinoma of the colorectal cancer cells [1]. The clinical significance of a down regulation in RPN2 expression has been emphasized by several studies which led us to regard it as a point of focus in resistant colorectal cancer cells [18,25].
In colorectal malignant tumours having high level of RPN2 expression, RPN2 is involved in mediation of EGFR glycosylation. Glycosylated EGFR is then transported towards the cell surface through identification and localisation of glycochain. After binding of EGFR and ligands (EGF and TGF-α), glycosylated EGCF activated towards phosphorylated EGFR (p-EGFR), hence lead to the ERK activation and p-ERK enters in the nucleus for cell proliferation malignancy and promotion. However in colorectal non-malignant tumours having lower level of RPN2 expression (shRPN2), RPN2/EGFR/ERK signal impairment occurrence causes decrease growth of cells (Figure 1) [33].
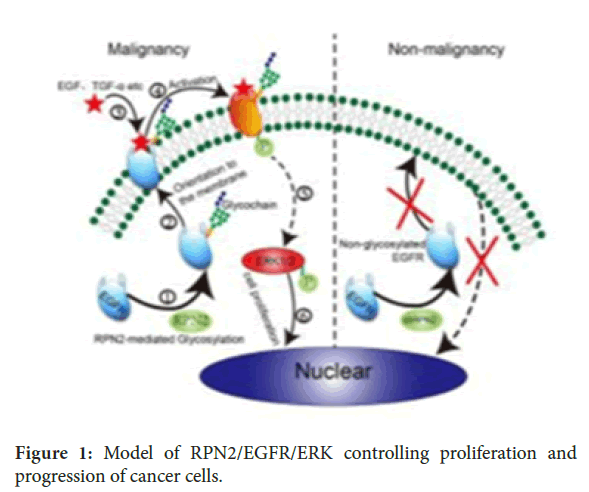
Figure 1. Model of RPN2/EGFR/ERK controlling proliferation and progression of cancer cells.
Studies revealed the role of RPN2 on regulation of EGFR expression through EGFR/ERK signaling pathway where by glycosyation process is modulated and in turn lead to controlling and suppression of cell proliferation and induction of apoptosis this implies RPN2 plays crucial part on controlling the overexpression and aberrant roles of EGFR found on colorectal cancer [33-35]. EGFR expression has been identified as target for treatment for several types of cancer due to it is high expression is found on various tumours such as colorectal cancer[36-38] therefore it is important to focus on EGFR inhibition for achievement of colorectal cancer treatment. Resistance of many cancer cells include colorectal cancer to chemotheurapeutic agents has been a challenge for several decades however recent studies has shown the promising role of RPN2 functioning on EGFR expression in chemotherapeutic resistant cells [39,40]. This has resulted to development of monoclonal antibodies such as coteximab and paramamub to be used as alternative therapy to those resistant colorectal cancer cells [41].
Treatment and prognosis of metastatic cancer including colorectal cancer need a proper and reliable targeted biomarker. Identification of markers like RPN2 provides a platform for treatment even in chemotheraupeutic resistant colorectal cancer cells [42]. CRC as a fourth leading cause of cancer death has been associated with chemotherapeutic resistance to various anticancer agents includes 5-flourouracil this has necessitate the identification of proper biomarker to targets its management [43-45]. Recently studies has shown RPN2 as a novel biomarker which is highly expressed on CRC and it involved in rate of cell proliferation and spreading of cancer cells [33,46]. The mechanism of action of RPN2 is through EGFR/ERK pathway whereby EGFR expression is controlled through inhibition of glycosylation process by RPN2 [33]. Genomic studies through numbering and profiling has proved RPN2 to be an essential biomarker and a novel target in EGFR expression whereby resistant to chemotherapeutic agent can be altered and development of new treatment can be attained like in monoclonal antibodies which serve as alternative to resistant chemotherapeutic agents toward CRC and other related malignant tumours [47-49]. Endoplasmic Reticulum ER is major component of cell involved in protein synthesis, RPN II as component of ER remain determinant biomarker for cell cycle and proliferation, it is expression determine the state of the cell towards disease condition including CRC. As studies shows its high expression signify disease progression and a bases to measure the effectiveness of chemotherapeutic agents. Over expression of RPN II In chemotherapeutic resistant cancer cells has been identified and used as marker which demand the treatment strategies to be reviewed as indicated on docetaxel and cisplatin resistant cancer cells [18]. Studies have shown the importance of biomarkers for the effective treatment of resistant cancer cells where proliferation of RPN II and silencing has become of useful method to personalize chemotherapeutic resistant cancer cells whereby genomic profiling can be identified and institute specific therapy [50]. Gene alteration in colorectal cancer has been used as new quest for cancer drivers and biomarkers and helped to strengthen the use of RPN II and other marker on CRC resistant dugs and develop of current Novel therapies [51]. Currently where chemotherapeutic resistant is prevailing the use of proper biomarker is of paramount importance to ensure quality therapy and overcome side effects of the drugs. Over expression of RPN II and associated ER proteins such as EGFR are the major current biomarkers used in diagnosis of resistant chemotherapeutic drugs in CRC and other solid cancer in including breast cancer, lung cancer and gastric cancer. The mechanism of action of RPN II towards resistant cancer has been associated with its ability to over express on resistant cancer cells while become down regulated/ silenced if the drug does not confer resistance.
The CRC can arise from different causative factors including life style, infection and genetic predisposition factors. Modern developmental therapeutics requires that a new treatment to be the specific molecular, genetic or immunologic subtype, importantly, Currently studies accepted advances biomarker identification as predictor and provision of proper diagnostics for stratifying and subgrouping patients in improving the quality of clinical care in oncology largely considering this era of personalized treatment where management of cancer is shifting from a populationbased empirical 'one drug fits all treatment model, to a focused personalized approach where rational companion diagnostic tests support the drug's clinical utility by identifying the most responsive patient subgroup and relevant biomarkers. RPN II is current prognostic biomarker identified to be effective in predicting the CRC and chemotherapeutic resistance effects. Through it is crucial action in colorectal cancer and it is biomarker ability to other carcinoma like breast, oesophagus, osteosarcoma, RPN II stands as potential focus towards elucidation of novel diagnostic biomarker to both CRC and chemotherapeutic resistant cancer cells.
I appreciate the Department of Gastrointestinal Surgery staffs and colleagues for their support.
None
Received: 17-Jan-2020 Published: 01-Feb-2020
Copyright: 2020 Al Japairai, et al. This is an open access paper distributed under the Creative Commons Attribution License. Journal of Biology and Today's World is published by Lexis Publisher.