Research Article - (2023) Volume 12, Issue 6
Background: Blood loss relating to surgical procedures is difficult to predict. Inadvertent vascular injury or insidious loss over time can lead to post-surgical anemia and, in some instances, vascular collapse, cardiac arrest, and death. Replacement of lost blood can be achieved using allogenic donor sources (banked blood) or through preplanned collection and reinfusion of extracorporeal autologous blood collected by means of Cell Saver technologies or pre-surgical autologous donation. Unfortunately, these methodologies require anticipation of blood loss and proactive decisions made prior to the undertaking of a procedure. In addition to necessitating preplanning, donor blood products and Cell Saver technology are expensive, labor intensive, and are often limited by unavailable resources. Packed cell reinfusion also fails to address the need for coagulation factors and proteins while at the same time often increasing the patient’s acidotic state. The latter can worsen a patient’s shock related metabolic lactic acidosis, making physiologic resuscitation even more difficult. These issues, while ubiquitous in the civilian hospital setting are magnified in the setting of Combat Casualty Care (CCC) where field hospital resuscitation is hampered by a paucity of technology and transfusable blood products. Aside from the mortality or major morbidity resulting from unexpected blood loss, post procedure anemia, a condition that frequently affects post-surgical patients, has negative effects on recovery especially in individuals over the age of 65.
Methods: A proof of concept study was conducted to test a novel disposable three stage filtration device to determine: (1) If gravity fed passage of clotted bovine blood through the filter system would exclude thrombus and other particulate such that the filtered product was free of particulate matter, (2) If gravity fed passage of non-clotted porcine and human blood through the system caused red blood cell damage that could preclude safely returning the filtered blood to the donor animal as an autologous transfusion and (3) If gravity filtration altered the coagulation profile of filtered human blood when compared to unfiltered blood. For the porcine portion of the study, unfiltered (control) and filtered (experimental) blood from the same animal was tested for serum (Ca), serum (K), and Hematocrit (Hct), and was also examined micro-histologically to determine if cell morphology was altered by the filtration process. Elevations in ion concentrations in the post filtered sample was felt to be a reliable measure of potential filtration induced hemolysis as was microscopic evaluation of blood smears. For the human portion of the study unfiltered and filtered blood was studied for coagulation anomalies using PT, PTT and INR. RBC integrity was evaluated by measuring LDH, ALT, AST, total bilirubin, K, and Hct. Platelets count and RBC /platelet integrity on blood smear was also performed.
Results: (1) Results after the filtering of thrombosed bovine blood showed no residual thrombus in the final filtrate, (2) When the unfiltered (control) and filtered (experimental) porcine blood samples were compared, no differences in extracellular (Ca) or (K) were detected. Hct remained consistent between the two samples and micro-histologic analysis showed no evidence of red blood cell damage, (3) When unfiltered and filtered human blood was evaluated no differences in coagulation or other hematologic parameters were identified, (4) When unfiltered and filtered human blood was compared, all chemical markers for hemolysis aside from a single LDH level remained normal thus supporting the high likelihood of absent filter induced hemolysis.
Conclusion: Using a novel gravity fed filtration system, processing of a solution containing bovine thrombus and saline showed no residual thrombus in the final filtrate. Extracorporeal processing of extracted porcine and human blood using the same device showed no evidence of red blood cell injury or alteration of coagulation profile when unfiltered and filtered blood samples were compared. While further investigations are needed to determine short and long-term safety of infusion of autologous blood treated in this manner, this proof-of-concept study provides encouraging data that will guide further development of an inexpensive, mobile, disposable and technologically simple device that can be used in every surgical procedure (civilian and military) to recycle any volume of blood loss. Such technology will hopefully reduce health care expense by making autologous blood recycling cost-effective in developed and undeveloped nations.
Adoption of routine filtration and reinfusion may also reduce the incidence of post-surgical anemia, reduce the need for allogenic donor blood, reduce reliance on costly Cell Saver technologies and provide a level of safety that does not currently accompany every invasive procedure that carries the risk of blood loss. Applications in military and disaster settings will provide a degree of care that has long been reserved for established brick and mortar settings. It is our hope that simple recycling of procedural blood loss will establish a new standard of care that no longer considers nonrecycled blood loss a normal consequence of invasive procedures.
Trial Registration: This article does not report intervention results on human participants. Animal use was approved by the State University of New York at Buffalo, Canon Stroke and Vascular Research Center IRB # NSG07123N. Human blood was personally provided by the author (MH) and routine laboratory testing was performed.
Blood reinfusion • Autologous blood transfusion • Blood filtration • Combat casualty care • Military medicine • Blood loss
CCC: Combat Care Casualty; Ca: Calcium; K: Potassium; Hct: Hematocrit; PT: Prothrombin Time; PTT: Partial Thromboplastin Time; INR: International Normalized Ratio; RBC: Red Blood Cell; LDH: Lactate Dehydrogenase; ALT: Alanine Transaminase; ALT: Aspartate Transaminase; cc: Cubic Centimeters; mg: Milligrams; kg: Kilogram; g: Grams; dL: Deciliter; Hgb: Hemoglobin; PRBC: Packed Red Blood Cells; Rh: Rhesus factor; US: United States; $: US Dollars; in: Inches; cm: Centimeters; um: Micrometers; mm: Millimeters; OR: Operating Room; IV: Intravenous; BCT: Blood Component Therapy
In order to maximize success while mitigating risk one must anticipate and prepare for untoward events that stand in the way of task completion. Risk reduction and success rate maximization necessitate the expenditure of limited financial/natural resources, time, and effort. For this reason, when an entity undertakes an endeavor, it often evaluates the benefits and the costs associated with risk mitigation. The likelihood of an untoward event and the seriousness of the potential negative consequences of that event generally determine the degree of resources allotted towards risk reduction. The cost of mitigation in turn influences allotment of resources dedicated to such risk mitigation. Thus, we have the concept of the risk: Benefit and cost: Benefit ratios.
Now, consider the following surgical factoids:
• The average human contains (4500 cc to 5000 cc) of circulating blood (male 70 ml/kg vs female 65 mg/kg).
o Loss of over 14% of blood volume (650 cc to 700 cc) causes physical symptoms and vital sign changes
o Loss of 20% or more of blood volume can cause hypovolemic shock (900 cc to 1000 cc)
o Loss of over 50% of blood volume (2250 cc to 3500 cc) can cause cardiac arrest (without proper resuscitation)
• Caesarian section blood loss often exceeds 500 cc [1].
• 18%-70% of patients undergoing knee or hip replacement receive blood transfusions [2].
• 8% to 36% of spine surgery patients required perioperative blood transfusions; these transfusions were generally performed 7 days before to 30 days after surgery [3-6].
• Allogeneic blood transfusion may be an independent risk factor for bacterial infections in orthopedic surgery, which may result in higher morbidity and worse prognoses, particularly in elderly patients. This hypothesis is supported by several animal models. The same result was not observed when syngeneic blood was given [7,8].
• Pre-surgical and post-surgical anemia (Male Hct<41%; Hgb<13.5 g/dL // Female Hct<36%; Hgb<12 g/dL) significantly increases morbidity and mortality especially in individuals over the age of 65 [9].
• Hours and days following an invasive procedure, blood loss can continue from the surgical site as blood is collected by, and then discarded from, surgical drains. This is especially relevant to the placement of drains following planned surgery or trauma. These may include subgaleal scalp drains, chest tubes, pigtail catheters and/or peritoneal drains which may extract a large volume of blood that is freely flowing from manipulated and injured organs.
• Allogenic blood transfusions must match patient blood and Rh type and must take into consideration antibody incompatibilities. Such requirements at times make it difficult to locate compatible donors for a particular individual.
• Banked blood for transfusion is a scarce commodity as only 3% of Americans donate. According to the Red Cross, approximately 29,000 units of Red Blood Cells (RBC) are needed in the US/day which leads to frequent shortages.
• Blood is expensive with the average pint of banked blood costing $200- 300. The cost of transfusion can reach $3,000/pint when overhead such as storage, transport and administrative costs are calculated [10,11].
• Most blood banked blood comes in the form of Packed Red Blood Cells (PRBC) which is devoid of platelets, albumin, and clotting factors and which may be acidotic. Many physicians prefer replacing blood loss with whole blood which contains these impor tant components.
• Many invasive procedures are performed without high volume blood loss. In these instances, however, lower amounts of blood loss may lead to anemia, but not to acute life-threatening events. Although post-surgical anemia negatively influences post-surgical outcomes, the risks of allogenic blood transfusion (donated blood from another individual), the shortage of transfusable blood products, and the cost of blood make the risk: Benefit and cost: Benefit ratio of transfusion too great for management of anemia alone [12].
• When managing shock secondary to blood loss, it is often more advantageous to infuse whole blood (blood that contains RBC, platelets, clotting factors, and proteins) as opposed to individual blood components PRBC.
If physicians performing or supporting invasive procedures (surgeons, cardiologists, gastroenterologists, radiologists, anesthesiologists) anticipate the high likelihood of significant blood loss they can prospectively arrange for the availability of blood products or Cell Savers in order to intervene and reduce the risk of life-threatening anemia and/ or volume loss. Unfortunately, however, physicians cannot reliably predict procedural blood loss or, when they do, underestimate its extent. Khan opined that “bleeding is an unavoidable side effect of most surgical operations. There is a scarcity of literature that can provide an estimate of how much blood will be lost” [12]. Sudden uncontrolled bleeding or steady, ongoing, insidious intraoperative blood loss is a common occurrence. The surgeon and anesthesiologists often find themselves in a position where a blood transfusion is necessary or desired, but not available. Even more commonly, non-life-threatening procedural blood loss leads to post-surgical anemia and its attendant negative effects on recovery and outcome. Simply put, excess intra-procedural blood loss is often unexpected yet accepted as a necessary evil. If blood was available for transfusion, it would be administered. However, for the following reasons, in most cases, it is not.
• PRBC are expensive and, if not used, often go to waste. According to Rao, et al. the average unit cost for allogenic banked blood was $765- $2609 [11].
• Cell Saver equipment is expensive (>$1500 for hardware which does not include disposable items for each case) and labor intensive to operate. According to Rao, the average unit cost for washed post-operatively salvaged cells was $759-$1827 [11].
• Sourcing of PRBC and Whole Blood is difficult due to a paucity of donors and the difficulty in some cases of matching donors to recipients due to antibody incompatibilities.
• Surgical teams often accept the risk of unforeseen blood loss without prospectively planning for expedient intra-operative blood replacement.
Having PRBC, whole blood, or a Cell Saver in every operating room for all surgical cases would be ideal and analogous to having a fire extinguisher in every kitchen. (“You May Never Need It But, When You Do, You’re Glad You Have It”). In reality, however, fire extinguishers are easily sourced, safe, readily available, simple to operate, and inexpensive while blood products and Cell Savers are not. In order to make transfusable blood more readily available the benefit: Cost and benefit: Risk ratios must be ele vated.
As Khan noted above, surgeons often fail to prospectively predict life threatening intra-procedural blood loss and are willing to accept non-lifethreatening anemia as a consequence of many surgical procedures. This does not mean maintaining a normal Hct and Hgb are not desirable. It does, however, suggest that a better approach needs to be developed to manage routine and excessive intraoperative and post-operative blood loss. The ability to quickly, simply and inexpensively re-transfuse a patient’s filtered unwashed lost blood back into their circulation can provide for an improvement in current practices. It is our belief that immediate blood collection and reinfusion can be achieved using a single, inexpensive, disposable, technologically simple filtration system that has a fixed cost of operation whether it is used to service and rein fuse 1 unit or 20 units of unwashed autologous blood.
A portable, inexpensive, technologically simple, and disposable filter system was designed and tested to determine its ability to safely and quickly process extracorporeal blood prior to patient re-infusion. Composed of three serially connected in-line collection chambers each housing a variable pore sized removable and rinseable filter (Filter A: 2 mm; Filter B: 250 microns; Filter C: 40 microns), the device shown in Figures 1a and 1b uses gravity feed to strain extracorporeally collected blood prior to autologous reinfusion. Figure 1c contains an embedded video of the device being used to filter blood during an endovascular thrombectomy procedure. Figure 1d illustrates one proposed iteration of the system used to filter and collect surgical blood loss. Figure 1e illustrates another proposed iteration of the system wherein blood loss is filtered and immediately reinfused into the patient using an inline pump.
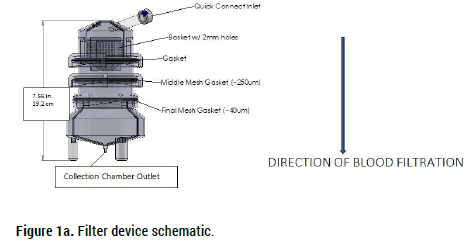
Figure 1a. Filter device schematic.
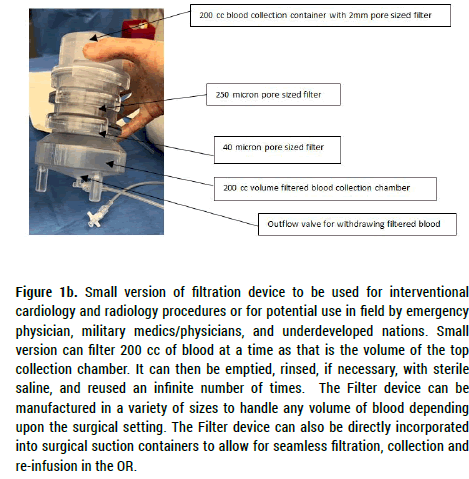
Figure 1b. Small version of filtration device to be used for interventional cardiology and radiology procedures or for potential use in field by emergency
physician, military medics/physicians, and underdeveloped nations. Small
version can filter 200 cc of blood at a time as that is the volume of the top
collection chamber. It can then be emptied, rinsed, if necessary, with sterile
saline, and reused an infinite number of times. The Filter device can be
manufactured in a variety of sizes to handle any volume of blood depending
upon the surgical setting. The Filter device can also be directly incorporated
into surgical suction containers to allow for seamless filtration, collection and
re-infusion in the OR.

Figure 1c. Video of small version filtration device being demonstrated on blood aspirated in angiography suite as part of a thrombectomy procedure. (Filtered blood WAS NOT rein fused into patient).
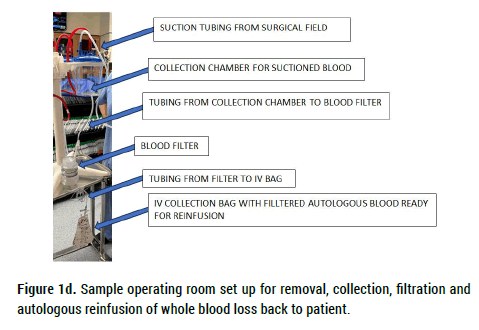
Figure 1d. Sample operating room set up for removal, collection, filtration and autologous reinfusion of whole blood loss back to patient.

Figure 1e. Figure depicts concept for real time reinfusion of blood loss from surgical field immediately and directly back into patient using a closed system and in line pump; Schematic concept for real time surgical blood loss collection, filtration and reinfusion; A: Surgeon at surgical field uses suction in right hand to collect blood loss; B: Blood exits surgical field via suction tubing; C: Empties into suction container; D: Blood flows from base of suction container through tubing; E: Blood flows from base of suction container through tubing and blood filter system; F: Filtered autologous whole blood flows from filters filtered blood collection chamber; G: Filtered autologous whole blood flows from filters filtered blood collection chamber and then back into patient via IV tubing; H: Filtered autologous whole blood flows from filters filtered blood collection chamber and then back into patient; I: Filtered whole blood can re-enter patient via IV tubing utilizing gravity flow or utilizing IV tubing that runs through a pump. Note: No IV bag collection of blood prior to reinfusion.
Study Part 1: Evaluation of filter system’s ability to extract thrombus from a 60 cc thrombus/saline mixture
Thirty (30 cc) of thrombosed bovine blood (30 cc) was combined with saline and drawn into a 60 cc syringe. This mixture was then introduced to the device’s top collection chamber via the Quick Connect Inlet. Under gravity feed, the mixture was serially strained by the three in line filters. The final filtrate was visually examined for particulate matter.
Study Part 2: Evaluation of filter system’s effects on RBC integrity and morphology
In order to evaluate the filter’s potential effect on processed blood cells, 50 cc of untreated porcine blood was collected and divided into 10 cc and 40 cc samples. The 10 cc sample remained unfiltered and was micro histologically evaluated to determine RBC integrity. Serum (Ca) and Serum (K) along with Hct% were also determined. The 40 cc samples were processed through the gravity fed filter (Figure 1). Filtered blood was then removed and evaluated in an identical fashion to the 10 cc unfiltered sample.
This process was repeated using human blood. Unfiltered and filtered samples were evaluated for LDH, AST, ALT, total bilirubin, Albumin, (K), Hct, and Platelet count. Blood smear was microscopically visualized for evidence of cell damage and plasma obtained from a spun sample of whole blood was checked for evidence of hemolysis.
Study Part 3: Evaluation of filter system’s effects on coagulation profile
In order to evaluate the filter’s potential effect on filtered blood’s coagulation profile, human blood was collected and divided into two samples. Sample #1 remained unfiltered and tested for PT, PTT and INR values. Sample #2 was passed through the filter device and testing was repeated for PT, PTT, and INR values.
Study Part 1: Evaluation of filter system’s ability to extract thrombus from a 60cc thrombus/saline mixture
Following passage of the thrombus/saline mixture through the threetiered filter system the final filtrate contained no visible thrombotic or particulate matter (Figure 2).
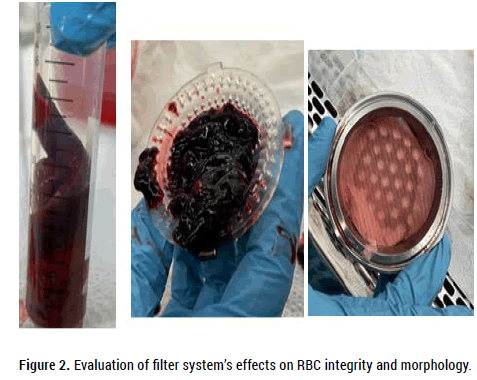
Figure 2. Evaluation of filter system’s effects on RBC integrity and morphology.
Study Part 2: Evaluation of filter system’s effects on RBC integrity and morphology
Table 1a shows serum (K), serum (Ca) and Hct results for the unfiltered and filtered porcine blood samples. There were no differences between the unfiltered control and the filtered sample in terms of serum (K), serum (Ca) and Hct. Differences in platelet counts were artifactual due to platelet clumping in the unfiltered sample. Micro histologic evaluation (blood smear examination) of both unfiltered and filtered samples also showed no differences in RBC morphology. According to the blood smear review “Erythrocytes and platelets display no significant morphologic abnormalities”. No schistocytes (RBC fragments) were identified.
| Serum (K) mg/dl (Normal) | Serum (Ca) mg/dl (Normal) | HCT% (Normal) | Platelets% (Normal) | |
|---|---|---|---|---|
| Control (unfiltered) | 4.2 (3.5-5.5) | 10.0 (7.2-11) | 33 (28-40) | 152 (200-800 x 1000) |
| Sample (filtered) | 4.3 (3.5-5.5) | 10.1 (7.2-11.5) | 32 (28-40) | 181 (200-800 x 1000) |
Table 1a. Data collected for filtered and unfiltered porcine blood sample.
Table 1b shows serum (K), total bilirubin, LDH, Hct, Platelet count, and visual plasma appearance of unfiltered and filtered human blood. There were no differences between the unfiltered and filtered samples aside from elevated LDH in the human filtered sample and an elevated PTT in the human unfiltered sample. Microscopic evaluation of the blood smear showed no evidence for RBC or platelet injury. No schistocytes were noted in either sample.
| Control unfiltered (Normal) | Sample filtered (Normal) | |
|---|---|---|
| LDH | 171 U/L (120-246) | 323 U/L (120-246) |
| Total bilirubin | 0.5 mg/dL (0.2-1.1) | 0.4 mg/dL (0.2-1.1) |
| AST | 27 U/L (0-34) | 32 U/L (0-34) |
| ALT | 43 U/L (10-49) | 44 U/L (10-49) |
| Albumin | 4.9 g/dl (3.2-4.8) | 4.6 (3.2-4.8) |
| K | 4.7 mmol/L (3.5-5.1) | 4.6 mmol/L (3.5-5.1) |
| HCT | 49.4% (40-51) | 47% (40-51) |
| Platelet | 286 K/uL (150-400) | 285 K/uL (150-400) |
| Blood smear | Normal | Normal |
Table 1b. Data collected for filtered and unfiltered human blood sample.
Study Part 3: Evaluation of filter system’s effects on human blood coagulation profile
Table 2 shows PT, PTT, INR and TEG results for an unfiltered and filtered human blood sample. There were no differences between the unfiltered control and the filtered samples.
| Tested sample | PT | PTT | INR |
|---|---|---|---|
| Control (unfiltered) | 10.5 (10.2-12.9) | 52 (25-36) | 0.93 |
| Sample (filtered) | 10.5 (10.2-12.9) | 31 (25-36) | 0.93 |
Table 2. Coagulation data for filtered and unfiltered human blood sample.
This proof-of-concept study sought to determine:
• If a novel three-tiered gravity fed blood filtration system could remove bovine thrombus from a thrombus/saline mixture and provide a filtrate free of particulate matter.
• If the filtration system could filter porcine and human blood without injuring RBC and platelets. This would be determined by measuring pre and post-filtration serum Ca, K, LDH, AST, ALT, and total bilirubin.
• If filtration altered the coagulation measurements of filtered compared to unfiltered human blood from the same donor.
The results posted show virtually no differences between the unfiltered and filtered porcine or human blood samples both histologically and chemically. Known chemical markers for hemolysis include AST, ALT, LDH, K and total bilirubin. In this study’s pre and post-filtration measurements of porcine and human blood only LDH was elevated in the human post filtration sample. All other markers for hemolysis remained normal.
The above indicate that thrombus filtration was efficient, no likely RBC or platelet damage was caused by the gravity fed filtration device and no alteration of the normal coagulation cascade was induced by the filtration process [13]. This report’s findings appear to indicate that gravity filtration can be used to safely remove particles (thrombus, foreign bodies) larger than 40 microns in diameter prior to recycling autologous extracorporeal blood for patient reinfusion.
Recycling of autologous blood may provide a simple solution for replacing blood loss and maintaining patient blood volume and Hct in situations where excessive blood loss is unexpected or reluctantly accepted by responsible physicians. It may also lead to a paradigm shift in the management of previously considered routine blood loss during surgical procedures and improve care in combat casualty care settings.
While the above discussion focuses on the benefits of recycling of autologous blood loss secondary to elective or emergent surgical procedures in the civilian setting, collection/filtration/reinfusion of autologous blood presents considerable opportunities in military (Combat Casualty Care; CCC) and civilian trauma/shock settings where the use of whole blood vs. individual blood components is advantageous. Whole blood has the advantage of providing immediate infusion of red blood cells, plasma volume, and clotting factors as it contains all of the preferred components of the recommended 1:1:1 ratio of trauma resuscitation that is traditionally used with Blood Component Therapy (BCT). Whole blood, therefore, is preferably used to treat patients who need all blood components such as those who have sustained significant blood loss due to trauma or surgery. Martin D. Zielinski, MD Chief of Trauma and Acute Care Surgery at Baylor College of Medicine in Houston, Texas concurs when he wrote that "most severely injured trauma patients are coagulopathic rather than anemic, which leads to worsening bleeding and worsening clinical situations.” Transfusion guidelines, which were extrapolated from the elective surgery setting, failed to account for the coagulopathy associated with hemorrhagic shock. Whole blood transfusions help ameliorate these conditions while PRBC alone do not.
Loss of blood, the “elixir of life” has been the accepted norm for far too long. On site recycling of autologous whole blood can both reduce our reliance on donated blood products and at the same time reduce our historical tolerance of post procedural anemia. This shift may improve surgical outcomes, save lives in situations when banked blood is not available, and reduce health care costs. As such, on site blood filtration and reinfusion can have far reaching civilian and military applications both inside and outside the surgical theater. To make this possible, the device shown in Figure 1 can be manufactured in a variety of sizes. Chamber volumes can be increased to accommodate open surgical procedures and decreased for catheter based interventional radiologic and interventional cardiac based procedures (i.e. functional heart, aspiration thrombectomy). Smaller portable devices can also be constructed for use in the field by Emergency Physicians as well as civilian and military Medics/Nurses.
We acknowledge that while this proof-of-concept study’s finding are encouraging, more blood samples need to be analyzed. Future testing should include:
• Determination of pre and post filtration free Hgb (fHB) and platelet function measurements [14]. Filtered blood should also be read ministered to the donor animal to evaluate the potential physiologic effects of autologous reinfusion. Further work is planned.
• In order to evaluate surgical scenario cell integrity and coagulation properties, blood suctioned from surgical fields such as those encountered in complex spinal surgery that generate a large particulate load and which involve large amounts of tissue trauma (cautery heat, crush, high speed drills) should be evaluated before and after filtration to assess the quality of the filtered product prior to reinfusion. This investigative path is especially important in view of recent work that suggests blood recovered from cell savers during spine surgery contains greater than expected numbers of damaged reticulocytes and platelets [15].
If filtration and reinfusion of autologous blood is safe, simple and inexpensive, we posit three simple questions:
• Why as a society would we continue to put so much emphasis on recycling paper goods and plastics and so little on recycling and reinfusing a person’s own blood no matter how much they lose during a procedure?
• Why would we begin any surgical procedure without being prepared and capable of immediately re-infusing expected and unexpected excessive blood loss?
• Regardless of volume, why would we dispose of a patient’s lost blood when this valuable commodity can be simply, safely, quickly and inexpensively filtered and reinfused?
If future studies confirm the safety and efficacy of simple gravity blood filtration and re-infusion, we see no reason why gravity filtration and autologous blood re-infusion should not become the norm for all surgical procedures no matter the expected and measured blood loss. We also anticipate filtration being used for re-infusion of eligible blood collected from post-surgical drains and chest tubes. Collection, filtration and reinfusion may be especially applicable in military theaters where the need to re-transfuse an injured individual’s blood often occurs in a setting where blood banked blood is difficult or impossible to acquire. Medics and physicians may be able to insert chest tubes and drains in front line settings, collect blood and reinfuse it thus maintaining adequate resuscitative blood volume until definitive care can be rendered. Battle field surgical procedures may also benefit from autologous blood reinfusion when blood banked blood is not available.
Ethics approval
This article does not report intervention results on human participants. Animal use was approved by the State University of New York at Buffalo, Canon Stroke and Vascular Research Center IRB #NSG07123N. Human blood was personally provided by the author (MH) and routine laboratory testing was performed. IRB approval was not required for testing of the author’s (MH) blood. All data is publishable under the IRB Board guidelines and consent for use of blood test results from the author’s (MH) blood has been provided by MH.
Funding
Funding for this study was provided by Retriever Medical.
Availability of data and materials
All data sets have been provided in the Manuscript’s Results section. Any additional information regarding the tested device can be obtained through the corresponding author (MH).
Disclosures
Michael Horowitz, MD is a Founding Partner with equity holdings within Retriever Medical, LLC.
Benjamin Bobo is a Founding Partner with equity holdings within Retriever Medical, LLC.
Hieu Le is Vice President of Research and Development with equity holdings within Retriever Medical, LLC.
Brandon Repko, MD is Chief Science Officer with equity holdings within Retriever Medical, LLC.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Horowitz M, et al. Extra-Corporeal Processing of Bovine, Porcine and Human Blood in Preparation for Autologous Re-Infusion Using a Disposable Filtration System: Initial Proof of Concept Study and Potential Applications for Human Autologous Blood Reinfusion in the Civilian and Combat Casualty Care Settings. J Biol Todays World, 2023,12(6),001-006
Received: 25-Nov-2023, Manuscript No. JBTW-23-121508; Editor assigned: 29-Nov-2023, Pre QC No. JBTW-23-121508 (PQ); Reviewed: 13-Dec-2023, QC No. JBTW-23-121508; Revised: 20-Dec-2023, Manuscript No. JBTW-23-121508 (R); Published: 27-Dec-2023, DOI: 10.35248/2322-3308-12.6.001
Copyright: © 2023 Horowitz M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.