Review Article - (2021) Volume 10, Issue 5
In this article, we describe a highly efficient and reproducible method of Agrobacterium-mediated transformation protocol for several gene transformations (cry1Ab, rolB, and PhMV) in tomatoes. Tomato fruit is rich in minerals, essential amino acids, sugar, vitamin B, iron, phosphorus, and dietary fiber. Tomato Crop production has some major problem which includes early ripening, reduction in fruit shelf life and devastating diseases that caused by viruses, bacteria, fungi, and nematodes, etc. The study aims to use the Agrobacterium-mediated transformation method to improve the tomato shelf life and make tomato plants resistant to such types of diseases caused by bacteria, viruses, and fungi, etc. Tomato was transformed via Agrobacterium tumefaciens strain LBA4404 harbouring Cry1Ab synthetic gene for toxin proteins. And same strain was inserted with another CP of PhMV that cloned into the binary vector pBI 121 for resistance to PhMV infection, and strain GV3101 harbouring the binary vector pICBV19 for reducing the expression of vis1 gene. Tomato seed culture on MS media after sterilization with 75% ethanol and distilled water. Plantlet root formation was on different kanamycin MS mediums. Constricted plasmid vectors with a gene if interest allowed to be transferred into Agrobacterium tumefaciens using the freeze-thaw method. The engineered strain suspension was centrifuged and re-suspended with MS media. Tomato seeds were obtained, then 0.5 cm per cotyledon, and put into the Agrobacterium suspension and co-cultivated and incubated and a transgenic plant, analysis was carried out using PCR, real-time Reanalysis, Northern blot analysis, and statistical analysis. For tomato varieties, the protocol developed showed very high transformation efficiency. The optimized transformation process is simple, effective and no need for tobacco, Petunia, tomato suspension feeder layer, or acetosyringone as chemical transduction.
Agrobacterium transformation • Transgenic tomato • Genetic transformation • Hypocotyls • In vitro regeneration
Tomato is an important cultivated crop in the world that belongs to the family Solanaceae [1]. It was originated in the South American Andes. In the sixteenth century, the cultivated tomato was brought to Europe by the Spanish conquistadors and later spread from Europe to southern and eastern Asia, Africa, and the Middle East region. Tomato is an annual plant and can reach a height of over two meters. Tomato fruit contains a variety of compounds that are important for human health and for supporting a well-balanced diet. It is rich in minerals, essential amino acids, sugar, dietary fibers, vitamin B, iron, and phosphorus. It also contains several antioxidant properties such as lycopene and ascorbic acid [2]. Economically, tomato is very attractive for medium-scale farmers due to its high yield and relatively short duration crop. In 2008, globally, tomato production achieved about 136.230 million tonnes from an area of 4.837 million hectares and the average yield was 28.16 tonnes/ha [3]. In South America, the same plant individual can be harvested for several years in succession. The first harvest is possible 90-120 days after sowing or 45-55 days after flowering [2]. Tomato crop production is influenced by several biotic factors resulting in great economic losses [4]. The main problem is devastating diseases of tomatoes caused by viruses, bacteria, fungi, and nematodes, such as early blight disease or known as Alternaria leaf spots. It is a common disease caused by the fungus Alternaria tenuissima [5]. For example, Fusarium wilt disease is caused by the soil-dwelling fungus Fusarium oxysporum F. sp radicis-lycopersici [6]. An important problem is in the post-harvest storage process of tomatoes, namely abnormal ripening, and a reduction in fruit shelf life. An increase in the ambient temperature of more than 300°C will stimulate production and accumulation of toxic ROS (Reactive Oxygen Species) and induced abnormal ripening, lack of lycopene accumulation, and an increase in cell wall depolymerization enzymes which causing fruit tissues to soften prematurely and leading to up to 50% yield loss and a reduction in fruit shelf life [7].
Tomato is both an important food crop and serves as a model plant species that are used for various research investigations including understanding gene function. Transformation is commonly utilized to overcome several problems of tomato, in combination with all the extensive genetic and genomic resources available for tomatoes [8]. Agrobacterium tumefaciens has played a major role in the development of plant genetic engineering and the basic research in molecular biology, especially in tomato [9]. It accounts for about 80% of transgenic plants produced so far [9]. In addition, the transformed cells usually carry single or low copy number T-DNA integrated into their genome with less rearrangement, and very large DNA segments can be transformed into the plants. It seems that the improvement of Agrobacterium-mediated transformation can offer us many opportunities and prospects, which are the subjects of this article.
Agrobacterium method
The method of a journal entitled "Agrobacterium-mediated transformation of tomatoes (Lycopersicum escalated L.cv. Hezuo 908) with increased efficiency" aims to improve the gene transformation protocol using Agrobacterium as a transformation medium. The explants were used hypocotyl and cotyledons. According to Sun et al. the stages of research in using Agrobacterium as a transformation medium are as follows [3].
The first step in this research is the preparation of plant material, namely tomato seeds (Lycopersicum esculentum L.cv. Hezuo 908) obtained from the Biotechnology Institute, Shanghai Agricultural Academy, which are then sterilized with 75% ethanol and distilled water and then cultured on MS media. Next, Optimization of plant regeneration was done by selecting plants that had the best hypocotyl and cotyledons. Then the selected plants were given IAA to form roots and given different concentrations of kanamycin in the MS medium. In this study, Agrobacterium tumefacient strain LBA4404 was used as a gene transformer mediator, tomato expression vectors were the plasmid vector pBIN438-AV1 (i/r), pBIN438-AC1 (i/r), pBIN438-AC3 (i/r), and pBIN438-AV1-AC1-AC3 (i/r) which has been constructed in the laboratory. Plasmids were transferred into A. tumefacient using the freeze-thaw method. The engineered strain suspension was centrifuged and re-suspended with MS media, and the result of this process was later used as infectious bacteria.
After 10 days of culturing tomato seeds, hypocotyl and cotyledons will be obtained, then 0.5 cm per cotyledon seed is cut, and hypocotyl (7-10 mm) is cut which will be used as explants. Then the explants were put into the Agrobacterium suspension. Next, the explants drying process on a filter paper and transferred to co-cultivation media and incubated at different times. Furthermore, the explants were transferred to shoot inducing medium. Finally, the explants were transferred to the root induction medium for rooting. The rooted tomatoes were subjected to different analyses, as putative transgenic plants. Transfer-motion cycle, which was also assessed in this study, is the time from tomato seeds sterilization to rooted transgenic plants obtaining. To determine whether tomatoes have become a transgenic plant, analysis was carried out using PCR, real-time PCR analysis, Northern blot analysis, and statistical analysis.
According to the research journals, the methods and stages of the research were almost the same, namely the tomato explants used to use the hypocotyl and the cotyledons. To grow the cotyledons and hypocotyls of tomato plants which will later be used as explants in the Murashige Skok growth medium which libraries with IBA and IAA hormones to stimulate the growth of roots and shoots containing hypocotyl and epicotyl added that the stages of gene transformation into tomato plants using Agrobacterium tumefacient media also use the hypocotyl growth method from sterilized tomato plant seeds and then cultured on MS media, the hypocotyl that has been successfully grown and selected as explants will be inserted into the Agrobacterium suspension [10,11]. Tumefacient containing recombinant vector plasmid. Apart from using the Agrobacterium tumefacient strain, the mediator of gene transformation into tomato plants is also data using Agrobacterium rhizogenic [12].
In previous study using the same method principle succeeded in inserting the Cry1Ab synthetic gene as a gene for producing toxin proteins that will be expressed in tomato plants so that they can poison tomato- eating pests. The same analysis is used as other research journals, namely the PCR method and southern blot analysis to select tomato plants that were successfully inserted by the Cry1Ab synthetic gene. The results indicated that 100% of the pest larvae died 4-5 days after eating transgenic tomato plants [13].
Agrobacterium gene transfer in tomato
Agrobacterium tumefacienstransformation: Agrobacterium tumefaciens are gram-positive soil bacteria which are phytopathogenic. Bacteria naturally can transfer their DNA pathogens which are then known as T-DNA (DNA transfer) in the plant genome and crown gall [14]. There are three important genetic components involved in the process of tumor formation. The first chromosomal virulence (chv) which are found on agrobacterium chromosomes and function in the attachment of bacteria to plant cells, secondly, a group of virulence (vir) genes contained in large (200 kb) Ti plasmids (Tumor inducing) that play a role in inducing the transfer and integration of T-DNA. The third component is the T-DNA region located on the Ti plasmid. The T-DNA region is limited by LB (Left Border) and RB (Right Border) containing important genes for Agrobacterium. The ability of this agrobacterium which can be used to insert genes [15], the transfer process of T-DNA from Agrobacterium tumefaciens to plant cells is divided into 2 stages, the process of stages in bacteria and stages in plant cells. The stage in the bacterial cell is regulated by the activity of the vir gene. The basis of the transformation using Agrobacterium tumefacient is that there are various proteins encoded by the vir gene that is used for the transformation process. virA and virG proteins are two components that function as sensors for signal transduction [16].
The process by which A. rhizogenic transfers its T-DNA is initiated upon detection of certain phenolic and sugar compounds that are produced from the wounding site of a potential host plant. Phenolic chemicals are perceived by the virA sensory protein leading to autophosphorylation of the VirA protein and subsequent transphosphorylation of the VirG protein, which results in the activation of the vir operon [17]. These virulence regions encode different Vir proteins that play a role in the transfer process. VirD2 and VirD1 proteins play an important role in the process of cutting single-strand T-DNA from Ti plasmids. Then VirD2binds covalently to the 5 'end of the detached DNA. This molecule is then released to the plant cell via a channel. This channel is formed by the virB protein. This channel is also used to export virE protein. The process that occurs in plant cells is the combination of virE with single-stranded T-DNAa and virD molecules to form a T-complex structure. VirD2 and VirE2 protein carry T-DNA into the nucleus through Nuclear Pore Complexes (NPC) and then in the nucleus, the T-DNA is integrated into the plant genome cell (Figure 1) [18].
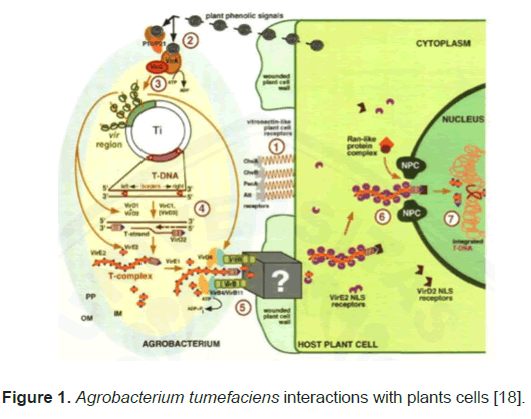
Figure 1: Agrobacterium tumefaciens interactions with plants cells [18].
Agrobacterium-mediated genetic transformation in Tomato (Lycopersicon esculentum): The use of Agrobacterium to insert various genes in tomatoes is adapted to the problems faced by farmers in agricultural land to produce maximum tomato production. One of the challenges faced in tomato cultivation is a disease that can reduce crop yields. A robust Agrobacterium-mediated genetic transformation system was developed for aphid resistance in tomato cultivars Jamila and Tomaland. A significant reduction in aphid fecundity was achieved by plant- mediated aphid-RNA silencing using the T-452 construct containing two Ace 1 fragments in the reverse and forward orientations that dramatically decreased aphid population growth and lead to a substantial reduction in agricultural losses by developing disease resistance [19]. Agrobacterium- mediated tomato transformation is also successfully used to produce transgenic tomato variety Rio Grande plants expressing Agrobacterium rhizogenes rolB gene that showed significantly higher (24%-225%) ascorbic acid content. In addition, rolB expressing tomato fruits exhibited an 11% to 58% increase in total phenolic content. Two rolB lines RB IX and RB VII exhibited a maximum increase of 58% (21 mg/g) and 53% (20 mg/g), respectively, in their phenolic contents. Highest relative increase (26%) in antioxidant activity was exhibited by RBI (EC50=536 mg/mL) followed by RB VII (EC50=558 mg/mL) and RB IX (EC50=564 mg/mL) which showed 23% and 22% increase in their antioxidant capacities, respectively [11].
Moreover, Agrobacterium tumefaciens strain LBA 4404 is one of the favorite strains using by the researcher [7,20]. An Agrobacterium-mediated tomato cultivar Hezuo 908 transformation system was established, which provides a baseline for efficient insertion of a gene into this cultivar using Agrobacterium tumefaciens strain LBA 4404, and the transformation frequency reached 40% per explant. The highest transformation efficiency was shown by the cotyledon (45%) and hypocotyl (33%) infected with bacterial suspension for 20 and 30 min, respectively, and co-cultivated for 2 d. At 10 d after culture, some buds turned green, whereas others turned white. At 30 d after culture under light illumination, green buds proliferated and elongated (Figures 2a and 2b). Green buds were cut and transferred to the shoot proliferation medium and then transferred to the root induction medium. After rooting (Figure 2c), the plantlets were transferred to the greenhouse and grown in soil (Figures 2d and 2e) [3]. This strain not only raising transformation frequency but also can be used to produce virus-resistant plants. Agrobacteriumtumefaciensstrain LBA 4404 was inserted with The CP of PhMV that cloned into the binary vector PBI 121 (Clontech, USA) at the Sma I and Sst I sites give successful development of transgenic tomato plants cultivar Pusa ruby expressing the coat protein gene of PhMV that show resistance to PhMV infection [1].
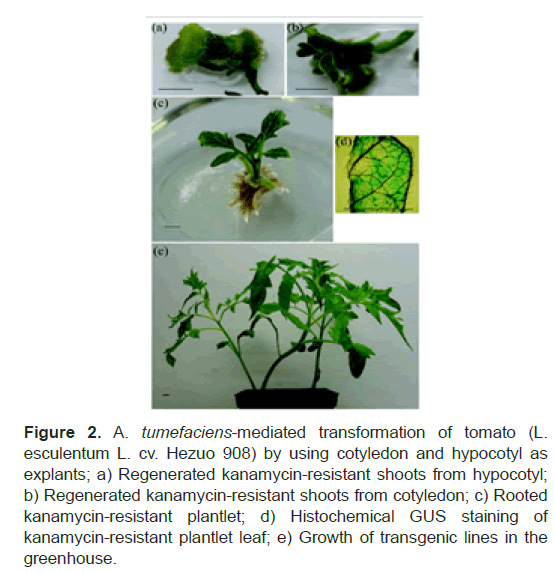
Figure 2: A. tumefaciens-mediated transformation of tomato (L. esculentum L. cv. Hezuo 908) by using cotyledon and hypocotyl as explants; a) Regenerated kanamycin-resistant shoots from hypocotyl; b) Regenerated kanamycin-resistant shoots from cotyledon; c) Rooted kanamycin-resistant plantlet; d) Histochemical GUS staining of kanamycin-resistant plantlet leaf; e) Growth of transgenic lines in the greenhouse.
Gene introduction using Agrobacterium is now the most popular method of transformation and has the potential to alter the manipulated traits in plant breeding programs. Agrobacterium-mediated plant transformation is the preferred method for commercial biotech product development because of its simplicity and propensity to produce cleaner methods. The improved protocol was shown to be effective in transforming other tomato varieties. It is proven that the Agrobacterium- mediated plant transformation from various studies has generally been successful. The optimized protocol is simple and reproducible and may be adapted for other tomato cultivars. However, there remain many challenges to be addressed. Transformation of economically important plant species, which are highly recalcitrant to Agrobacterium-mediated plant transformation, is a great challenge. The use of Agrobacterium for site-directed recombination to avoid random T-DNA integration and to deliver T-DNA efficiently into the plant nucleus is another challenging task. Moreover, stable integration of the transgene and consistent inheritance in further generations without loss or alteration of expression must be established.
Agrobacterium gene expression patterns and how they may be altered during co-cultivation of bacterium and different plant species need to be investigated. The plastid genetic transformation by Agrobacterium is another important aspect to be considered. The final challenge involves the genetic transformation of animal and plant pathogenic fungi and the human and animal cells where Agrobacterium offers an exciting possibility. The detailed analysis of the plant genes involved in Agrobacterium- mediated transformation will contribute to a better understanding of molecular events, such as cell communication, intracellular molecular transport, DNA repair, and recombination, which occur during the process of Agrobacterium-mediated transformation and may help to broaden the range of elite cultivars with improved transformation efficiency of economically important crops. The development of new technologies for Agrobacterium-mediated transformation will be of great value in plant science and will promote further research and development in the scope of agriculture.
Citation: Jawad, et al. Agrobacterium-Mediated Transformation Improvement in Tomato. J Biol Today's World, 2021, 10(5), 001-003.
Received: 20-Jul-2021 Published: 10-Aug-2021, DOI: 10.35248/2322-3308.21.10.017
Copyright: © 2021 Jawad, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.