Review Article - (2023) Volume 12, Issue 4
Hyperlipidemia and its associated health risks have posed a great threat to the mankind, globally. Hyperlipidemia refers to a group of inherited and acquired; chronic and progressive metabolic disorder wherein, the plasma or serum contains higher than 90 percentile Low Density Lipoprotein (LDL-C), Total Cholesterol (TC), Triglyceride levels (TG), or lipoprotein levels in comparison to the general population, or 10 percentile lower values of HDL in comparison to the general populace. Although, numerous drugs are available in the market for mitigating hyperlipidemia, however they come with side effects and high cost. The mankind has shifted their focus on alternate, safe and cost effective remedies to regulate serum lipid levels. Probiotics with bile salt hydrolase activity are one among the safe alternative remedies to treat such metabolic disorders. Bile salt hydrolase, present in gut microorganisms is the key enzyme responsible for the efficient lowering of serum cholesterol levels by the deconjugation of host derived conjugated bile acids into deconjugated forms that are readily expelled from the body. To make up for the lost bile acids, cholesterol is used up to synthesize bile acids, thereby lowering serum cholesterol levels. Apart from this, BSH activity provides defense to the BSH harboring microorganism from the hostile bile environment in GIT. This fact thus makes it important to list out the assessment of BSH activity while selecting probiotics. This review focuses on the role of BSH activity among probiotics and its role in mitigating hyperlipidemia.
Bile Salt Hydrolase (BSH) • Hypolipidemia • Microorganisms • Bile acids • Probiotics
Hyperlipidemia and its associated health risks have posed a big threat to global public health system. Hyperlipidemia refers to a group of inherited and acquired metabolic disorder. It is a chronic and progressive diseased condition in which the plasma or serum contains higher than 90 percentile Low Density Lipoprotein (LDL), total cholesterol, triglyceride levels, or lipoprotein levels when compared to the general population, or 10 percentile lower values of HDL in comparison to the general populace. Hyperlipidemia is associated with various health risks which includes cardiovascular diseases, cerebrovascular diseases, hypertension, atherosclerosis, metabolic syndrome, and many chronic diseases. In spite of the availability of innumerable lipid lowering pharmacological drugs, many patients prefer non-pharmacological agents for hyperlipidemia due to several reasons, which includes the side effects of lipid lowering drugs, high cost associated with the drugs and preference for natural/alternative remedies. Probiotics are the most common dietary supplement and one of the most promising alternative remedy for hyperlipidemia. Further, dietary preventative strategies being crucial for the general populace, including individuals who do not exhibit signs of dyslipidemia, probiotics have an added benefit towards human health. However, as per the International Scientific Association for Probiotics and Prebiotics (ISAPP), in order to be selected as a suitable probiotic microorganism, the intended probiotics must go through a series of in vitro/in vivo trials to assess its health claims and safety [1,2].
Over the last few decades, probiotics have gained much popularity owing to the continuous increase in the scientific data that supports their positive effects on human health. Probiotics are live microorganisms that, when consumed in sufficient quantity, contribute to the well-being of an individual beyond innate basic nutrition. Generally, the most commonly known probiotics used as supplements and additives in food are the members of lactic acid bacteria and Bifidobacteria. The beneficial health claims of probiotic consumption confirmed through numerous in vitro studies and clinical trials includes, enhancement of gastrointestinal health, metabolism of lactose, cancer suppression, immune modulatory functions, serum cholesterol reduction and various other ailments [3].
Probiotic microorganisms employ numerous mechanisms in order to exert its hypocholesterolemic activity. They may assimilate and incorporate cholesterol into the host’s dividing somatic cells; incorporate cholesterol into their cell membranes; bind cholesterol to their cell surfaces, which prevents the formation of cholesterol micelles in the intestine; or assimilate cholesterol during growth, which lowers the amount of cholesterol available for intestinal absorption. Further, the deconjugation of bile salts by the action of bacterial Bile Salt Hydrolase (BSH) has been delineated as one of the key mechanism related to the hypocholesterolemic activity of probiotics. Thus, the World Health Organization (WHO) has proposed that BSH activity must be considered as one of the key criterion for the selection of probiotics. This review shall focus only on the BSH activity of probiotics and its associated hypolipidemic activity [4].
Bile acids and their role in cholesterol regulation
Primary bile acids, a water soluble byproduct of cholesterol are molecules of saturated hydroxylated C-24 sterols produced by the liver hepatocytes via a series of enzymatic reactions involving 17 genes. Cholic Acid (CA) and Chenodeoxycholic Acid (CDCA) are the chief primary bile acids produced in the rodents and humans. Upon synthesis, about 99% primary bile acids are subjected to conjugation with amino acids taurine or glycine that is then released into the bile. This conjugation step brings about the reduction of the pKa and enhances the solubility of the bile acids, rendering it unfit for passive passage through cell membranes. They are then actively secreted via the canaliculi into the gallbladder beginning the process of bile formation. Accumulation and concentration of bile occurs in the gallbladder until they are released into the duodenum upon food consumption. As a consequence of the secretion of bile into the duodenum, bile enters the enterohepatic circulation. This enterohepatic circulation proves to be very efficient since about 95% of the bile acids are reabsorbed and recirculated, while only about 5% are lost in the faeces. Bile acids thus lost in the faeces are regained by their de novo synthesis from cholesterol. This in turn brings about the regulation of cholesterol levels in the host. It is noteworthy that the production of bile acids from cholesterol and their loss in the faeces marks the major route for the removal of excess cholesterol. However, in the 1960's, it was first noted that germ free animals accumulated cholesterol in larger quantities and at a faster pace than their conventional counterparts, which explains that the intestinal microorganisms take part in accelerating the metabolism of cholesterol via increased bile acid elimination.
Apart from cholesterol regulating potentialities of the bile acids, they also possess other important physiological functions, which include regulation of vitamin absorption, liver function, fat digestion and antimicrobial activities. They are considered as biological detergents that plays role in the host defense system against commensal gut inhabitants and enteric pathogenic microorganisms. The detergent like, bacteriostatic and bactericidal activity of the bile acids may disrupt the bacterial cell wall, which is made up of lipids and fatty acids. However, some gut commensals and pathogens can resist the deleterious effects of bile [5].
Deconjugation of bile acids by Bile Salt Hydrolase (BSH)
Modifications of the primary bile acids are an important defense mechanism of the microorganism and also the chief mechanism involved in the cholesterol lowering activity of probiotics. The biotransformation of the host’s primary bile acids into secondary bile acids is brought about by the gut microorganisms. About 5% of the unabsorbed bile acids are subjected to chemical diversification by three main microbial pathways i.e. deconjugation, dehydroxylation and dehydrogenation in the distal ileum, caecal and colonic part of the host. Deconjugation is the result of bile salt hydrolase, an enzyme belonging to the choloylglycine hydrolase enzyme family; prevalent among the gut microorganisms and lacking in the eukaryotes. Also, reported in probiotic microorganisms that are the natural inhabitants of the human digestive tract, BSH plays its role in the deconjugation of glycine and taurine bile acids by catalyzing the hydrolysis of amide bond in conjugated bile salts to release free bile acids (mainly cholic and chenodeoxycholic) in the enterohepatic circulation [6]. The deconjugated bile salts thus formed possess lower solubility, are less efficiently reabsorbed from the intestinal lumen and are therefore expelled in the faeces. In order to maintain the homeostatic balance, cholesterol is required for the synthesis of new bile acids, thereby bringing about the reduction of serum cholesterol levels. Also, the deconjugated form of bile salts exhibit better adherence to the microorganisms and dietary fibers thereby enhancing the ability of bile salt removal. The mechanism of cholesterol reduction by means of probiotic BSH mediated deconjugation of bile salt has been briefly given in Figure 1.
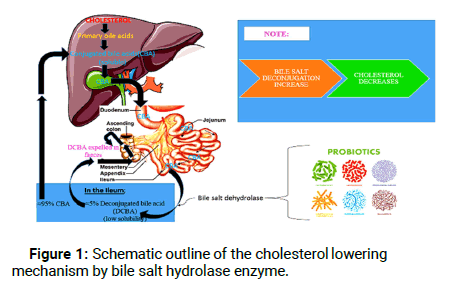
Figure 1: Schematic outline of the cholesterol lowering mechanism by bile salt hydrolase enzyme.
The physiological functions associated with BSHs are as follows; (i) BSHs offer nutritional benefit by liberating amino acids, which can be used as a carbon and nitrogen source. Also, BSHs can act as an energy source by using taurine as an electron acceptor; (ii) BSHs also help in the incorporation of cholesterol and the components of bile into the bacterial membranes, thereby helping in the maintenance of the integrity and fluidity of the cell membrane; (iii) BSHs also help in detoxification by lessening the detergent properties of bile acids. In addition to this, BSHs improves the colonization of the microorganism within the GI tract, however, this ability is strain specific. This fact is very well elucidated by the metagenomic analysis conducted by Jones, et al., which demonstrated that gut microorganisms possessing BSH activity exhibited enhanced survival in the GIT and better resistance to conjugated bile acids. The physiological functions of BSHs have been tabulated in Table 1 [7].
| Function of BSH activity on the host |
|---|
| Serum cholesterol reduction. |
| Survival of probiotic microorganisms in the GIT adds to the overall health of the host. |
| Function of BSH activity on the probiotic microorganism |
| Deconjugation of bile salt releases taurine, which may be utilized by the microorganism as an electron acceptor, thereby exhibiting enhanced growth in medium containing taurine/taurine conjugated salts. |
| Deconjugation of bile salt releases amino acids, which in turn can act as a source of carbon, nitrogen and energy. |
| BSH mediated incorporation of bile/cholesterol into the bacterial cell membrane helps maintain the integrity and fluidity of the bacterial cell membrane thereby conferring dominance over BSH negative pathogens. |
| Bile detoxification by forming weaker deconjugated counterparts. |
| Improves colonization of the probiotics in the GIT. |
Table 1. Functional attributes of BSH.
BSH enzyme in probiotic bacteria
Unlike gram negative commensals, gram positive ones have been found to show BSH activity and their genome homolog. This comes with an exception from Stenotrophomonas, Brucella and the two strains of Bacteroides. As per the metagenomic analysis, the percentage of BSH present in the phylum Actinobacteria, Firmicutes and Bacteroidetes are 8.9%, 30% and 14.4%, respectively. Moreover, the BSH activity has been identified and extensively studied in various probiotic bacteria belonging to the genera Lactobacillus, Bifidobacterium, and Enterococcus. A first study reporting BSH activity in yeast has also been reported by Hernandez-Gomez, et al. The BSH activity of commercial probiotic yeast Saccharomyces cerevisiae var. boulardii CNCM I-745, isolated from Floratil® capsules, studied by Hernandez-Gomez, et al. showed 57%, 100% and 63% deconjugation action against taurocholate, sodium glycodeoxycholate and taurodeoxycholate, respectively. This study suggested that the supplements of S. cerevisiae var. boulardii could possibly lower serum cholesterol levels. In addition to this, a study by Jones, et al. has also revealed that the archaeal species Methanobrevibacter smithii and Methanospherastadmanae, belonging to the human gut microbiota also possess a BSH gene capable of degrading glyco- and tauro conjugated bile acids [8].
The presence of BSH gene has been observed in probiotics isolated from several fermented products. Pradhan, et al. have found BSH gene in Pediococcus pentosaceus SMB13-1 and Enterococcus faecium BPB11 isolated from traditionally prepared dry starters of the Eastern Himalayas. Likewise, in a study conducted on lactobacilli isolated from the two fermented drinks (water kefir and braga) showed the presence of BSH genes. The two Lactobacillus plantarum strains, BR9 and CR1 were the ones that harboured the BSH gene. Similarly the BSH gene was observed in L. plantarum strain S1.30 and SL2.7 isolated from dadhi, naturally fermented Indonesian milk.
In yet another study, Oner and Aydas studied the BSH activity of Lactobacillus and Bifidobacterium strains isolated from the faeces of a month old healthy breast fed infants. The highest enzyme activity i.e., 1.76 ± 0.23 U/mg protein and 1.42 ± 0.11 U/mg protein were observed in Bifidobacterium breve A26 and Lactobacillus plantarum LA3, respectively. Upon evaluation of sodium taurocholate deconjugation, the strains possessing highest BSH activity also showed high deconjugation capability. Thus, this analysis is suggestive of the correlation between BSH activity and the deconjugation ability. It also throws light on the ability of these strains to find role in cholesterol reduction.
Begley, et al. examined several potential probiotic bacterial strains like for e.g. Lactobacillus plantarum WCFS1 and found more than one BSH homolog in their genomes. They also found that BSH gene regions were not identical for all the strains. Also, in case of multiple BSH genes, the BSH gene regions were not located in the same region of the chromosomes [9].
In an evaluation conducted by Ren, et al. the functionality of four BSH genes from Lactobacillus plantarum ST-III, cloned in Escherichia coli were studied. The outcome of the result suggested the activity of the four BSH proteins in the deconjugation of tauro and glycol conjugated bile acids. Moreover, out of the four BSH proteins i.e. BSH1, BSH2, BSH3, and BSH4, the BSH activity towards glycol conjugated bile acid was found to be greater than that of the other proteins. This finding reveals the substrate specificity of BSH1. The over expression study of BSH protein revealed the molecular weights of the BSH protein BSH1, BSH2, BSH3, and BSH4 to be as follows; 37 kDa, 39 kDa, 37 kDa, and 36 kDa.
Rani, et al. studied the characteristics of 326 amino acid encoding BSH gene from Lactobacillus gasseri FR4. Through the SDS-PAGE and western blot examination the molecular weight of the BSH encoding gene was found to be 37 kDa. Similar to the findings of Ren et al. this study also revealed greater deconjugation of glycol conjugated bile acid by Lactobacillus gasseri associated BSH gene (LgBSH). Furthermore, the analysis of the inhibitory mechanism of LgBSH gene revealed that Riboflavin could inhibit the LgBSH gene by 98.31% and penicillin V could inhibit it by 97.84%.
In a particular study conducted by Kim et al. the BSH gene of Bifidobacterium bifidum ATCC11863 was characterized. The molecular mass of this gene was found to be 35 kDa by SDS polyacrylamide gel electrophoresis. When the substrate specificity of the BSH gene was studied, greater hydrolysis of glycol conjugated bile acid was reported, similar to the findings of Ren, et al. and Rani, et al. The molecular weight of BSH genes of few probiotic microorganisms has been tabulated in Table 2.
| Microorganism | Source of isolation | Molecular weight of the BSH gene |
|---|---|---|
| Lactobacillus plantarum ST-III | Chinese pickles | 37 kDa, 39 kDa, 37 kDa, and 36 kDa |
| Lactobacillus gasseri FR4 | GIT of chickens | 37 kDa |
| B. bifidum (ATCC11863), B. infantis KL412, B. longum (ATCC15708), B. longum KL507 and B. longum KL515 | - | 35 kDa |
| Bifidobacterium bifidum (ATCC 29521) | - | 35 kDa |
| B. longum SBT2928 | Human faeces | 37 kDa |
| B.bifidum (ATCC11863) | - | 35 kDa |
Table 2. Molecular weight of BSH gene reported from some probiotic isolates.
Clinical evidence supporting the role of BSH in hyperlipidemia
Microorganism targeted hypocholesterolaemic remedies have caught more attention as of late. In an evaluation conducted by Huo, et al. cholesterol reducing mechanism of Lactobacillus delbrueckii was assessed in a pig model. For this study, 12 barrows were grouped into two different groups; one group was fed with corn soybean meal supplemented with 0.1% of L. delbrueckii (5 × 1010 CFU/g, Con+LD) while the other group was fed with only corn soybean meal. As per the outcomes of the result it was found out that the group supplemented with BSH positive L. delbrueckii showed alleviated levels of serum Total Cholesterol (TC), Low Density Lipoprotein (LDL-C) and Triglycerides (TG) in comparison to the control group. Besides this, greater bile deconjugation by the L. delbrueckii supplementation, which thus affected serum and hepatic bile acid composition, was noticed. The findings also revealed that the ideal alteration by the L. delbrueckii supplementation enhanced the deconjugation of bile acids and thereby it’s fecal excretion.
In a study conducted by Wang, et al. two BSH positive Lactobacillus casei pWQH0, Lactobacillus plantarum AR113 and a BSH negative Lactobacillus casei LC2W were assessed for their cholesterol lowering property in vivo using 4 weeks old male C57BL/6J mice. 38 experimental C57BL/6J mice were grouped into five different groups namely, High Cholesterol Diet (HCD), AR113, pWQH01, LC2W and control groups. All the groups except the control groups were fed with high fat diet. In addition to this, AR113, pWQH01, LC2W groups were also fed with 0.2 ml of saline mixed with 1 × 109 CFU of L. plantarum AR113, L. casei pWQH01 and L. casei LC2W, respectively once a day on a daily basis. Their findings revealed that the groups fed with BSH positive strains (AR113 and pWQH01) could significantly alleviate the levels of Body Weight (BW), serum Total Cholesterol (TC), Low Density Lipoprotein Cholesterol (LDL-C) levels and Atherogenic Index (AI), while those fed with the BSH negative strain (LC2W) had poor ability to alleviate the same. Thus, this study suggests the possible role of BSH in mitigating cholesterol levels in the serum by lowering cholesterol absorption and enhancing cholesterol metabolism [10].
BSH activity and hypocholesterolaemic ability of probiotic Enterococcus strains has been reported by various authors. Likewise, a study by Singhal, et al. has reported the isolation of Enterococcus faecium harboring BSH gene, which could lower cholesterol in vitro However, out of the five isolated Enterococcus strains (LR2, LR3, ER5, LR13, and VB1) only LR2 showed BSH activity in the BSH phenotypic assay but, PCR amplification and sequence analysis confirmed the presence of BSH gene in all the other strains as well. Also, it was observed that all the strains possessed similar amino acid sequences of BSH, which suggests that these proteins are highly conserved in E. faecium. Besides this, all the strains showed significant reduction (>50%) of cholesterol in vitro probiotics with BSH activity are regarded capable of lowering serum cholesterol. This fact is in par with a study conducted by Huang, et al. They studied the BSH activity and anti-obesity effect of probiotic Lactobacillus plantarum TCI378, isolated from Korean Kimchi. Their findings revealed that the BSH activity of TCI378 was capable of deconjugating taurine conjugated bile acid (TDCA). In addition to this, in vitro analysis for assessing the cholesterol reduction potential of TCI378 showed a reduction in the cholesterol concentration to 216 μg/mL, 48% lower than that of the control. Thus, the results of this study suggested that the BSH activity of probiotic TCI378 was capable of playing its role in lowering serum cholesterol levels.
An in vitro analysis and theoretical assessment of the BSH activity and cholesterol regulation conducted by Smet, et al. throws light upon the use of BSH positive probiotics as an alternative remedy for hypercholesterolaemia. In this study, the BSH activity of LP80 wild type and BSH overproducing LP80 (pCBH1) were assessed. It was observed that LP80 (pCBH1) was capable of hydrolyzingglyco conjugated deoxycholate (GDCA) more readily than tauro conjugated deoxycholate (TDCA), suggesting the substrate specific role of BSH. Also, it was noted that the GDCA hydrolyzing capacity of the wild type strain was during its exponential growth phase while LP80 (pCBH1) immediately started the hydrolysis, which highlights that the BSH activity of strains may depend on growth. However, the hydrolysis of TDCA by LP80 (pCBH1) started only after the hydrolysis of GDCA, indicating the substrate preferentiality of BSH. To further validate their work, the BSH activity of LP80 (pCBH1) in pure culture was compared to that in mixed cultures by mimicking the microbial environment of the human small intestine. The outcome of the result revealed that the BSH activity of control experiment was very low in such environment that mimics the human GIT, while the supplementation of LP80 (pCBH1) significantly increased the BSH activity. It was also revealed that the BSH activity of LP80 (pCBH1) remained unaffected in the presence of the normal GIT microorganisms. As per the data obtained via these experiments and also the proven fact that a relationship exists between bile salt and cholesterol metabolism, it was thus proposed that a daily consumption of 250 ml yogurt supplemented with Log10 9.50 CFU/ml of the BSH overproducing LP80 (pCBH1) strain might significantly mitigate the problems of severe hypocholesterolaemia.
In an in vitro study conducted by Ghatani, et al. BSH positive Enterococcus lactis YY1 and BSH negative Enterococcus durans HS03 were assessed for their hypocholesterolaemic activity using Sprague Dawley (SD) rats. The SD rats were divided into five groups namely, Normal Control (ND), probiotic strain Enterococcus durans HS03 and High Cholesterol Diet (HCD1), probiotic strain Enterococcus lactis YY1 and High Cholesterol Diet (HCD2), and a combination of both strains and High Cholesterol Diet (HCD3) and Negative Control (HCD). The groups HCD1, HCD2 and HCD3, those that were fed with probiotic strains showed 22.55%, 6.67%, and 31.06% decrease in their serum cholesterol levels, respectively. Also, on analysis of the TG and VLDL-C the decreased concentrations were found to be 25.39%, 26.3%, and 33.21% for HCD1, HCD2 and HCD3, respectively. The reduction in LDL-cholesterol was found to be 33.66%, 28.50%, and 35.87%, respectively. Moreover, an increased levels of HDL was observed; 38.32% for HCD1, 47.9% for HCD2, and 41.92% for HCD3. Their findings suggested that both the Enterococcus strains could efficiently lower blood cholesterol levels.
In a study conducted by Parvez, et al. The BSH mediated deconjugation of taurocholate and oxgall by Bifidobacterium bifidum NRRL-1976 was qualitatively assessed. It was observed that NRRL-1976 showed better deconjugation of taurocholate rather than oxgall. Furthermore, when the cholesterol lowering property of the strain was evaluated, it was found that partial removal of cholesterol was seen when the NRRL-1976 cells were washed with phosphate buffer at pH 7, while the remaining cholesterol was withdrawn from the cells. This finding revealed that the NRRL-1976 cells assisted in cholesterol removal from growth medium by the process of bacterial assimilation and precipitation of the cholesterol.
The changes in the lifestyle and dietary habits of humans have alleviated the incidence of hyperlipidemia worldwide. Although, it is well-known that the human body has the ability to regulate its serum lipid levels by the de novo synthesis of bile acids from cholesterol precursors yet, this process is not as efficient as those of the gut microorganisms harboring BSH enzyme. BSH, an enzyme lacking in the eukaryotes mediates the deconjugation of soluble forms of conjugated bile acids to insoluble deconjugated forms. The deconjugated forms possessing low solubility, facilitates removal of bile acid from the human body. To make up for the lost bile acid pool, the cholesterol in the body is used up thereby alleviating the problems of hyperlipidemia. Apart from the cholesterol lowering role of BSH, it also plays active role in providing bile salt resistance and energy to the microorganism, thereby making them fit to survive in the human GIT. Thus, the assessment of BSH has also been listed out as one of the key criteria for the selection of probiotics. The preference of mankind for safe and alternative remedies to treat hyperlipidemia besides the conventional pharmacological agent is at a rise. Probiotics, harboring BSH could possibly become a safe panacea to mitigate hyperlipidemia.
Though, numerous probiotics belonging to the genera Lactobacillus, Bifidobacterium, and Enterococcus has been assessed for their BSH activity and its role in hyperlipidemia, yet there is much scope for revealing the BSH activity of probiotics belonging to the other genera as well. Not only this, an in depth study of the mechanisms of microbial transformation of BSH and also the mechanism of action of BSH at the molecular level could help reveal the potential of many BSH positive probiotics, thereby facilitating its use in treating hyperlipidemia. Also, the mechanism by which the BSH positive microorganisms protect the host from the ill effects of de conjugated bile acids needs to be clarified. Furthermore, there is a need to carry out a detailed research on the effects of the substrate specificity of different BSH genes and its possible role, if any on the microorganism and the host.
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
Citation: Ghatani K . "Bile Salt Hydrolase Activity of Probiotics and their Role in Hypolipidemia". J Biol Todays World, 2023, 12(1), 1-4.
Received: 29-Jul-2022, Manuscript No. JBTW-22-70703; Editor assigned: 31-Jul-2022, Pre QC No. JBTW-22-70703 (PQ); Reviewed: 14-Aug-2022, QC No. JBTW-22-70703; Revised: 29-Dec-2022, Manuscript No. JBTW-22-70703 (R); Published: 06-Jan-2023, DOI: 10.35248/2322-3308.12.1.006
Copyright: © 2023 Ghatani K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.