Research Article - (2020) Volume 9, Issue 12
Till now numerous anti-microbials are accessible, yet some pathogenic creatures are demonstrating protection from existing anti-infection agents. So it is required to scan new anti-infection agents for opposing pathogenic life forms. Actinomycetes are excellent asset for delivering optional metabolites that is anti-microbials; they are Streptomycin, Erythromycin, and Tetracycline. Actinomycetes are accessible in both marine and earthbound environment. From earthly biological system disengagement of new strain Actinomycetes is exceptionally troublesome contrasted and marine environment. Marine environment is a best asset for some strains of actinomycetes, since it is unexplored biological system having a great many various kinds of organisms. Upto 1% of marine actinomycetes were separated, yet at the same time we need to investigate more for finding new organisms. The majority of the disengaged marine microorganisms are from Streptomyces class (70%), marine growths (20%), Bacillus spp. (7%) and other microbes (1%-2%). 40 isolates are obtained from sample, among them 8 isolates are selected from primary screening for antibacterial activity. Out of 8, 6 isolates showed antimicrobial activity against 4 test organisms. 2 isolates showed activity in secondary screening. S2 isolate showed broad antibacterial activity.
Actinomycetes • Antibacteria
Most of the secondary metabolites are widely recognized from Actinomycetes including many antimicrobials such as streptomycin, erythromycin and tetracycline, with original and ingenious structures and potent biological activities. Thus actinomycetes are considered to be a potent resource for new lead compounds in drug development. In the exploration of marine derived actinomycetes as a source of antitumour compounds.
Actinomycetes are virtually sources of novel compounds with many therapeutic applications and hold a prominent position due to their diversity and proven ability to produce novel bioactive compounds. There are more than 22,000 known microbial secondary metabolites, of which 70% are produced by actinomycetes, 20% from fungi, 7% from Bacillus spp. and 1%-2% by other bacteria. Among the actinomycetes, more than 55% of antibiotics approximately 10,000 unknown antibiotics are produced by Streptomycetes group.
Sample collection
Two marine samples were collected in different regions along the coast of Bay of Bengal at Sagar Nagar, Visakhapatnam at a depth of 1 meter. All the samples were collected in bottles, maintained with sea water and transported to the laboratory for the isolation of Marine Actinomycetes (Table 1). The isolated colonies were screened for antibacterial activity.
| Sample | Place | Sediment texture and character |
|---|---|---|
| 1 | Sagar Nagar | Fine powdered sample |
Table 1. Source, place and description of marine sediment samples.
Macroscopic and microscopic characteristics
The isolates were examined for cultural characteristics like colony colour, texture and pigmentation. Purity of isolates was checked by Gram’s staining. Microscopic characters like Gram positive/Gram negative, cell morphology, presence and spores were examined.
Aerial mass colour: The colour of the mature sporulating aerial mycelium was recorded in a simple way (white, red, grey, green, blue). When the aerial mass colour were recorded. If the aerial mass colour of a strain to be studied shows intermediate tints, then also, both the colour series are noted.
Melonoid pigments: The grouping was made on the production of melanoid pigments (i.e., greenish brown, brownish black, pigment modified by other colors) on the medium. The strains were grouped as melanoid pigment produced (+) and not produced (-).
Reverse side pigments: The strains were divided into two groups, according to their ability to produce characteristic pigments on the reverse side of the colony, namely distinctive (+) and non-distinctive (-). In case, a colour with low chroma such as pale yellow, olive or yellowish brown occurs, it is incubated in the latter group (-).
Soluble pigments: The strains were divided into two groups by their ability to produce soluble pigments other than melanin, namely, produced (+) and not produced (-). The colour was recorded (Red, orange, green, yellow, blue and violet) colour of aerial mycelium, melanoid pigments, soluble pigments and spore chain morphology of ABT 206 was observed.
Screening of isolates for antimicrobial activity
All the isolates were screened for antibacterial activity. Four bacteria cultures were used as test organisms. The test organisms used in the present study were produced from the National Collection of Industrial Microorganisms (NCIM), Pune, India (Table 2).
| S.No | Bacteria | Gram staining |
|---|---|---|
| 1. | Bacillus cereus | Gram positive |
| 2. | Bacillus subtilis | Gram positive |
| 3 | Escherichia coli | Gram negative |
| 4. | Staphylococcus aureus | Gram positive |
Table 2. List of test organisms used in the study.
Two stages of screening were done to test the antimicrobial activity of the isolates as explained follow.
Primary screening
The actinomycetes isolates were initially screened for antibacterial activity by Cross streak method. A combination of SCA and Nutrient agar media in 1:1 ratio was used for this study and the medium was designated as SCNA. 100 ml of SCNA medium was prepared, autoclaved, cooled, poured into petri plates and was allowed to solidify. The plates were inoculated with each isolate by streaking them as a single straight line at the centre and were incubated at 28°C for 2 to 5 days to develop growth. The plates were then cross streaked with test organism’s perpendicular to the isolates and incubated for 24 h to observe for inhibition of growth of the test organisms near the isolate. Control SCNA medium having test organisms without marine was kept to check the antimicrobial activity. Isolates that exhibit a broad spectrum of antibacterial activity were selected for secondary screening (Cup plate method or Agar overlay method or disc diffusion method).
Secondary screening
Isolates that showed positive results in preliminary screening were selected for secondary screening by Cup plate method.
Antimicrobial extract preparation: Well sporulated isolates (7-10 days old) were used for the antibacterial production studies. Five ml of sterile water with 2 drops of tween was transferred aseptically into each slant and the growth of the isolate on the surface of the medium was scrapped with sterile inoculating needle and transferred each into 45 ml of production medium and incubated at 28°C on a rotatory shaker at 180 rpm for 5 days. Then the cells were disrupted, filtered and filtrate was transferred into 2 ml of centrifuge tube and centrifuged at 1000 rpm for 20 min, at 4°C and clear culture filtrate was separated. The clear supernatant was used for antibacterial assay using cup plate method. The antibacterial activity against the bacterial test organisms were tested on nutrient agar medium (Table 3).
| Constituent | Concentration (g/l) |
|---|---|
| Sucrose | 20 |
| Malt extract | 10 |
| Yeast extract | 4 |
| Dipotassium hygrogen phosphate | 5 |
| Sodium chloride | 2.5 |
| Zinc sulfate | 0.04 |
| Calcium carbonate | 0.4 |
| pH | 7 |
Table 3. Composition of production medium (PMI).
Cup plate method: Antimicrobial activity was tested against five bacterial test organisms. Nutrient agar medium was autoclaved, cooled to 40°C. The test organisms were pour plated , allowed to solidify , kept aside for 2 h to solidify and then wells were made in each plate with the help of borers. 0.1 ml of antimicrobial extract each isolate was added to each well and the plates were incubated in refrigerator for 2h for diffusion of antimicrobial extract. The plates were incubated at 37°C to observe the zone of inhibition. The zones were measured and expressed in mm comparatively the isolate showed better activity and effective against four test organisms (Table 4).
| S.No | Equipment used |
|---|---|
| 1. | Autoclave |
| 2. | Cooling centrifuge |
| 3. | Digital pH meter |
| 4. | Digital weighing machine |
| 5. | Laminar air flow chamber |
| 6. | Hot air oven |
| 7. | Orbital shaker |
Table 4. List of equipments/insruments used in the study.
One marine sediment sample was collected in the coast of Bay of Bengal at Sagar nagar, Visakhapatnam at a depth of I meter. The marine sediment samples collected from sagar nagar, Visakhapatnam were selected for isolation.
Screening of isolates for antimicrobial activity
All the isolates were screened for antibacterial activity. Two stages of screening namely Preliminary screening by Cross-Streak method and Secondary screening by Cup-Plate method or disc diffusion method was done for their antimicrobial activity.
Preliminary screening: All the 40 isolates were used for preliminary screening, of which some showed antimicrobial activity.
Among all the isolates, S2 showed broad range of inhibition against bacterial test organisms Bacillus Subtilis, Bacillus cereus, Staphylococcus aureus and Escherichia coli. Zone of inhibition against test organisms were shown in Figures 1 and 2 (Table 5).
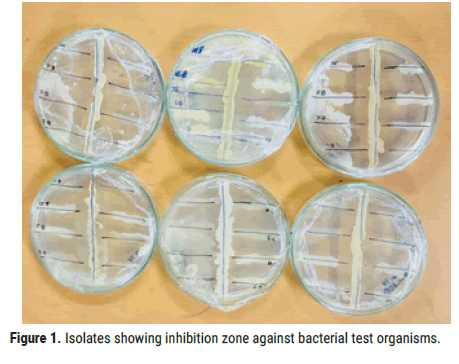
Figure 1. Isolates showing inhibition zone against bacterial test organisms.
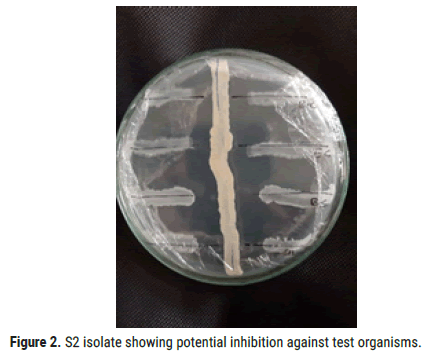
Figure 2. S2 isolate showing potential inhibition against test organisms.
| Isolate | E.coli | B.cereus | B.subtilis | S.aureus |
|---|---|---|---|---|
| S1 | - | - | - | - |
| S2 | +++ | ++ | ++ | + |
| S3 | + | - | + | ++ |
| S4 | - | - | - | - |
| S5 | ++ | + | + | - |
| S6 | + | + | - | ++ |
| S7 | + | + | ++ | +++ |
| S8 | ++ | - | + | ++ |
Table 5. Antimicrobial activity of isolates against bacterial test organisms by Cross-Streak method.
Secondary screening: All the isolates hat showed positive results in primary screening were inoculated into PM1 medium and incubated at 28°C for 7 days at 180 rpm. After 7th day, cells in the production media were disrupted, filtered and centrifuged at 10000 rpm for 10 minutes.
Filtrate was used for disc- diffusion method. Among 6 isolates from the primary screening, S2 showed antibacterial activity against test organisms (Figure 3).
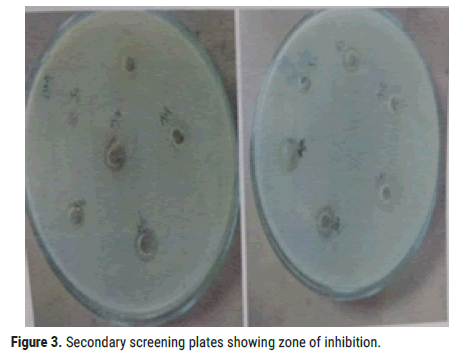
Figure 3. Secondary screening plates showing zone of inhibition.
For the treatment of multi-drug resistant pathogens, there has been increasing demand for effective antibiotics from soil Actinomycetes [1,2]. This is the reason we have tested the antibacterial activity of the isolates. The list of novel actinomycetes and products derived from poorly explored areas of the world, stresses the importance of investigating new habitats [3]. Both academic and industrial interest in marine microorganisms is on rise, in part because of the growing number of unique, biologically active secondary metabolites reported from marine bacteria [4]. Although initiated in the late 1970’s, natural drug discovery from the world’s oceans has been accelerated by the chemical uniqueness of marine organisms and the necessity to develop drugs active against the increasing number of resistant pathogens [5-8].
All the obtained isolates were screened for antimicrobial activity by primary screening (Cross streak plate) and secondary screening (disc diffusion method) against 4 bacterial pathogens which is gram positive bacteria: Bacillus cereus, Staphylococcus aureus, Bacillus subtilis; Gram negative: Escherichia coli. Six isolates showed antibacterial activity against bacterial test organisms in primary screening, they are S2, S3, S5, S6, S7, S8. When these six isolates were used for secondary screening, 2 isolates showed activity. This study clearly established that the isolated actinomycetes from Sagar nagar, Visakhapatnam, Andhra Pradesh, India. S2 showed antibacterial activity against Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, Escherichia coli.
Citation: Nirmala BS. Antibacterial Screening of Actinomycetes isolated along the coast Bay of Bengal, Visakhapatnam. J Biol Today's World, 2020, 9(12), 001-003.
Received: 15-Nov-2020 Published: 07-Dec-2020, DOI: 10.35248/2322-3308.20.9.244
Copyright: © 2020 Nirmala BS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.