Review Article - (2023) Volume 12, Issue 1
Actinomycetes are gram-positive bacteria detected in various terrestrial and aquatic environments. Many of them develop mycelia and exhibit complicated structural differentiation. Actinomycetes have been identified using a variety of methods, namely morphological, chemotaxonomic, and molecular techniques. Because of the extensively emerging antimicrobial-resistant organisms affecting human health and agriculture, as well as the increasing incidence of cancer, new drugs are urgently needed to combat different diseases and microbial infections. The majority of antimicrobials now in use are of natural origin. Among them, actinomycetes constitute a significant source of bioactive compounds used in drug development.
Actinomycetes provide almost 45% of the secondary metabolites, followed by fungi recording 38%, and other bacteria that produce only 17%. Secondary metabolites derived from actinomycetes exhibit a wide spectrum of biological activities targeting both the pathogens and the host. This review emphasizes the classification of diverse actinomycetes and lists their importance, with an emphasis on the secondary metabolites and their different biological activities.
Actinomycetes • Biotechnological value • Secondary metabolites • Antimicrobial • Therapeutic agent
Actinomycetes are a diverse group of microbes having filamentous mycelia. They are among the biggest taxonomic ranking between the main descendants identified nowadays within bacterial domain. Actinomycetes are gram-positive aerobic bacteria that have a high percentage of Guanine and Cytosine (GC) in their DNA. Currently, they are characterized by polyphasic method that uses morphological, chemotaxonomic, and molecular data to describe various strains [1]. Actinomycetes are abundant in natural settings and play a key role in a variety of metabolic and biological activities including the synthesis of extracellular enzymes. Moreover, actinomycetes produce nearly two-thirds of all naturally produced antibiotics employed in different fields (agriculture, medicine, and veterinary practice).
Amidst them, the Streptomyces genus produces the bulk therapeutic molecules recording almost 70% of total antimicrobials secreted by actinomycetes, where 75% of them are clinically available as antibiotics to treat several human infections.
Antimicrobial resistance has emerged as one of the major public health issues in the present time. This jeopardized the efficient prevention and curing of a wide variety of illnesses generated by different pathogens that are no longer sensitive to the frequently used medicines. The US center for disease control and prevention estimated that more than two million individuals get infected by antibiotic-resistant pathogens in the United States each year, with no less than 23,000 are dying because of these infections.
To combat the problem of resistance, ongoing research is being conducted to discover new medicinal substances. In this context, checking the natural environments seems a logical choice for the extraction of thousands of antimicrobials from different microbes. In addition to their antibacterial activity, natural compounds have an important role in creating advanced medicinal agents having antifungal, insecticidal, and anticancer activities with high efficiency [2].
Out of the prominent natural sources, microbes are the main reservoirs of beneficial molecules in medicine. More precisely, actinomycetes represent an outstanding group of bacteria that secrete different bioactive metabolites. Most actinomycetes spend more than half of their lifecycle as semi-dormant spores, mainly under stressful conditions. During stress, such as nutrient deprivation, competition occurs, which causes actinomycetes to release different secondary metabolites and form spores to survive.
In this review, we focus on offering an updated summary concerning the characterization of actinomycetes, as well as the major antibiotic classes and novel secondary metabolites that could represent promising therapeutic agents.
Morphological characteristics
Actinomycetes possess broad morphological varieties, majorly with regards to the absence or presence of aerial or substrate mycelia, their colors, the secretion of melanoid pigments, and the shape and form of their spores. The differentiation between the substrate and aerial mycelia is possible due to their dissimilarities in function and morphology. The substrate mycelium has a major role in absorbing nutrients from the medium to allow the growth of actinomycetes. It also has an extensive variety of colors. On the contrary, aerial mycelium is the hyphae produced by vegetative mycelium under stressful conditions. The main function of such mycelium is reproduction, in which spores develop on aerial hyphae. The hypha looks phasebright, refractive, and coarse [3].
Mycelial structure
Actinomycetes mycelia are similar to that of fungi, but smaller, and range from 0.4 m to 1.2 m in size. They can have a coccoid structure (like Micrococcus), rod-coccoid (like Arthrobacter), or a fragmenting hyphal form (like Nocardia spp.). Some actinomycetes form permanent and well differentiated branched mycelia (such as Frankia and Streptomyces species), others grow extended filaments on the surface of the medium, but do not develop genuine mycelia like Rhodococcus, while Corynebacterium does not develop any mycelia. In general, most Rhodococcus and Mycobacterium species do not possess aerial mycelia (Figure 1).
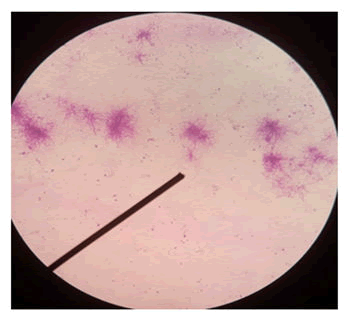
Figure 1: Branched mycelia shown under microscope Ã? 100.
Types of spores
Different morphological features of spores can be used to classify different actinomycetes species. Spores can have various shapes and surface characteristics. Spore shapes include rod-shaped, globose, reniform, doliform, allantoid, and ovoid forms. Also, surface ornamentation of spores varies to include parallel or irregular rugose, smooth, verrucose, hairy, warty, and spiny textures as shown in Figure 2.
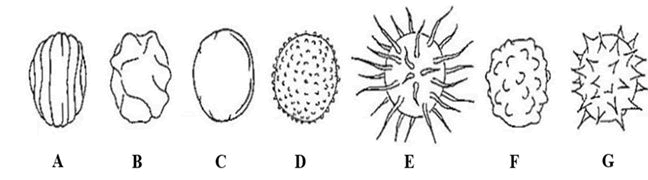
Figure 2: Surface ornamentation of spores. A) Parallel rugose; B) Irregular rugose; C) Smooth; D) Verrucose; E) Hairy; F) Warty; G) Spiny texture.
Spores can either form on aerial and/or substrate mycelium as sole spores, or chains of spores of varying lengths. In some instances, spores can be formed in unique vesicles called sporangium and supplied by a flagellum.
Monospore type is present in several genera including Micromonospora, Saccharomonospora, Thermoactinomyces, and Thermomonospora. Regarding spore chains, they can be divided into di-sporous, oligo-sporous, and poly-sporous chains. An example is Streptomyces genus that has a polysporous chain containing more than 50 spores. The aerial hyphae of Streptomyces can have one of several forms including rectiflexibiles spore chain, which can be straight or flexuous; retinaculiaperti spore chain having hooks with loops open or spirals with unusual shape and 1 twist to 4 twists; chain of spira spores in which spores are arranged in one of two spiral forms, either in a closed compact spiral form or in an open loose form; or verticillati type, with spore chains arranged in whorls. However, when spores are formed within sporangia, this can be further classified into two types: Sporangia developed on substrate mycelia, and others formed on aerial mycelia. For example, genus Ampullariella forms various structures of sporangia on substrate mycelium. On the other hand, Planomonospora, Planobispora, and Streptosporangium develop sporangia on aerial mycelium as shown in Figure 3.
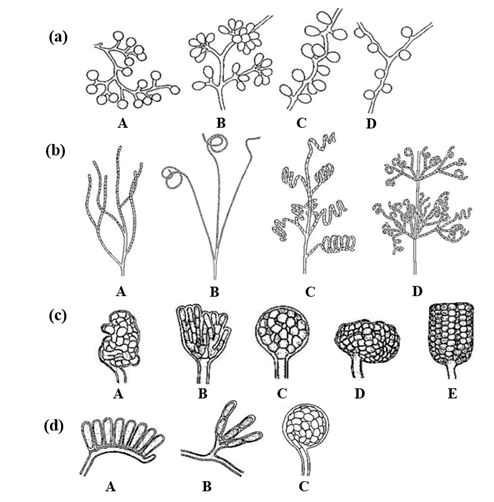
Figure 3: Spore types. (a) Production of a single spore by A) Micromonospora; B) Thermomonospora; C) Saccharomonospora; D) Thermoactinomyces; (b) Production of spores in chain A) Rectiflexibiles type; B) Retinaculiaperti; C) Spira spore chain; D) Verticillati type; (c) Production of spores in sporangia on substrate mycelium by Ampullariella of A) Irregular; B) Lobate; C) Globose; D) Sub-globose; E) Cylindrical shapes; (d) Production of spores in sporangia on aerial mycelium by A) Planomonospora; B) Planobispora; C) Streptosporangium.
Melanoid pigments
Melanins, also referred to as melanoid pigments, are polymers of various molecular structures with a crucial function in improving the competitiveness and survival of organisms. They also possess antioxidant activities and protect against ultraviolet radiations harmful to living beings. Melanoids are usually utilized in the fields of medicine, pharmacology and cosmetics. Depending on the strain, age of the culture, and the medium, actinomycetes produce different colors. It was shown that when same isolates were grown on different media (casein starch agar, glycerol asparagine agar, yeast extract-malt extract agar, starch yeast extract agar, tyrosine agar, and glycerol yeast extract agar), actinomycetes produced different pigments. On the other hand, when different isolates were grown on same media (casein starch agar), the colors of colonies varied [4].
Moreover, it was proven that the maximum melanin production was achieved at day 6 from incubation of actinomycetes. These pigments are useful in taxonomic studies. The production of pigments from some mycelia gives the medium and substrate mycelia different colors, which provides a significant clue in the detection of novel actinomycetes species. More specifically, substrate mycelia produce fat-soluble or water-soluble dyes. The former pigmentation colorizes the actinomycetes colony with the matching color, while the latter seeps into the surrounding medium thereby giving it a specific color.
Chemotaxonomic characteristics
Chemotaxonomy is utilizing the arrangement of chemical entities to categorize organisms based on the resemblance of their cellular chemistries. Several characteristics related to the composition and structure of peptidoglycans have been used to discriminate between actinomycetes species. These include the amino acid type in location three of the side chain of a tetrapeptide, the sugar content of peptidoglycans, and the existence of glycine in interpeptide bonds. Another important chemotaxonomic characteristic is the occurrence or lack of optical isomers of the amino acid 2,6-Diaminopimelic acid (DAP). DAP analysis is essential in studying the taxonomy of actinomycetes.
Based on major amino acids and sugar patterns, the actinomycetes cell wall can be split into four types. Type I is characterized by having L-DAP, glycine and no sugar as major constituents. Type II cell wall is comprised of meso-DAP, xylose, glycine and arabinose. Type III is composed of meso-DAP, and 3-O-methyl-D-galactose. And Type IV that have meso-DAP, galactose, and arabinose. Different groups may share a common DAP profile. Since the four groups of actinomycetes present different morphological characteristics, they belong to different families. Thus, when classifying actinomycetes, it is not enough to depend on DAP profiling, but on genotypic or phenotypic criteria as well [5].
Other chemotaxonomic indicators are used to identify actinomycetes genera. These include patterns of cellular fatty acid ranging from two (C2) to more than ninety (C90) atoms of carbon in bacteria. Only those ranging from C10 to C24 were used in the classification of actinomycetes. Menaquinone analysis is also used for characterizing various actinomycetes strains. In general, bacteria display different isoprenoid quinones, out of which menaquinones were mostly present in cell envelopes of actinomycetes. Genus Nocardia possesses cyclic menaquinones, while Pyrobaculum has fully saturated cyclic menaquinones. Moreover, phospholipid type that is distributed discontinuously in actinomycetes cytoplasm is used as a classification system to categorize different strains.
Eventually, analysis of sugar composition is used as a taxonomic marker. It divides actinomycetes into five groups: Group A includes strains having galactose and arabinose in their cell walls; group B contains 3-O-methyl-D-galactose in their cell walls; group C comprise no sugar markers; group D cell walls constitute of xylose and arabinose, and group E contain galactose and rhamnose in their cell walls [6].
Molecular features
Taxonomy of actinomycetes based on morphological and chemical identification was seen not suitable to distinguish various species of several genera. Thus, new molecular with phylogenetic strategies have been developed in the classification techniques. The advanced molecular approaches, improved the classification of actinomycetes. The phylogenetic relationships of different organisms, including bacteria, could be driven by comparing a conserved region of a genetic sequence. Lately, genetic analysis based on sequencing of 16S rDNA is being used for the identification of bacterial species with the aid of Polymerase Chain Reaction (PCR). And only a little work has been conducted on the 23S rRNA which is more complex, consisting of almost 3300 nucleotides.
The gene encoding for 16S rRNA is about 1550 nucleotides long and comprises both conserved and variable segments. This gene has adequate interspecific polymorphisms, which gives statistically significant measurements. The chosen primers are universal and are frequently complementary to the retained segments found at the start of the gene, and either at the end of the gene or the 540-nucleotide region. The region that is variable is utilized for sequencing in comparative taxonomy. Normally, when comparing the sequences of 16S rRNA, this permits to differentiate between all main phyla of bacteria at the level of genus. Also, it allows to group strains at diverse stages covering both species and subspecies levels [7].
Economic and biotechnological values
Actinomycetes are among the most useful prokaryotic organisms in commercial and biotechnological fields, where they are well recognized for producing significant secondary metabolites. Between different actinomycetes genera, Streptomyces, Actinoplanes, Micromonospora, Saccharopolyspora and Amycolatopsis constitute the main synthesizers of economically significant biomolecules. These include different medicinals including antibiotics, pesticides, plant and animal growth factors.
Examples of biologically active compounds synthesized by actinomycetes are shown in Table 1. Moreover, actinomycetes can prevent the reproduction of many plant pathogens including Agrobacterium tumefaciens (an agent causing crown gall disease), and Erwinia amylovora (bacteria causing fire blight to apple). Actinomycetes have also a role in biological buffering of soil, fixation of nitrogen, and degradation of complex compounds of high molecular mass such as hydrocarbons in soils that are polluted. They are also able to synthesize various vitamins, herbicides, nutritional materials, anti-parasitic substances, antibiotics, cosmetics, pesticides, and enzymes such as xylanase and cellulase utilized in the treatment of wastes [8].
| Compounds isolated from actinomycetes | Biological activity | Spectrum of activity |
|---|---|---|
| Chloramphenicol | Antibacterial | It has bacteriostatic effect against most pathogens but is bactericidal for Neisseria meningitides, and Streptococcus pneumoniae |
| Nystatin | Antifungal | It is active against broad spectrum of fungi including the genus Fusarium, Candida, Aspergillus, and Cryptococcus |
| Tunicamycin | Antiviral | Active against sindbis, vesicular stomatitis, semliki forest, herpes simplex, and encephalomyocarditis viruses |
| Rapamycin | Immunosuppressive | Rapamycin's powerful immunosuppressive activities are mediated in part by direct impacts on antigen-specific lymphocytes; nevertheless, rapamycin also affects adaptive immunity via actions on innate immune cells such as dendritic cells and macrophages |
| Streptozotocin | Diabetogenic | Induces type 1 diabetes mellitus |
| Meyastatin and lovastatin | Preventing high cholesterol | Lowers serum cholesterol and HDL content |
Table 1. Biologically active compounds synthesized by actinomycetes and their spectrum of activity.
Secondary metabolites production
A source of antibiotics: Antibiotics are biologically active compounds with various structures and mechanisms of action. The different antibacterial compounds isolated from various actinomycetes are represented in Table 2. The specificity of antibiotic activity is caused by the interruption of the essential processes exclusively related to bacterial survival. They majorly target each kind of bacterial processes such as synthesis of protein, DNA, and RNA, biosynthesis of cell wall, transport of electrons, the function of the membrane, germination, sporulation, and other different functions [9].
Antibiotics released by actinomycetes are divided into different chemical classes including macrolides, aminoglycosides, tetracyclines, ansamycins, angucyclines, streptogramins, chloramphenicol, beta lactams, as well as peptides, etc.
| Actinomycetes strain | Antibacterial compound | Spectrum of activity |
|---|---|---|
| Actinopolyspora | Erythrimycin | It is effective against gram-positive bacteria, including Listeria monocytogenes, Streptococcus pyogenes, Corynebacterium diphtheria, Streptococcus pneumoniae, Corynebacterium minutissimum, and Staphylococcus aureus. It also active against gram-negative bacteria including Neisseria gonorrhoeae, Bordetella pertussis, and Legionella pneumophila |
| Micromonospora rifamycinica | Rifamycin | It is particularly effective against mycobacteria |
| Verrucosispora AB-18-032 | Abyssomicins | Abyssomicin C is active against gram-positive bacteria including Enterococcus faecalis, vancomycin-resistant S. aureus, Bacillus thuringiensis, Micrococcus luteus, and MRSA |
| Streptomyces species | Chaxamycin D | It is effective against gram-positive bacteria including S. aureus |
| Streptomyces species QD01-2 | Gilvocarcin HE | It is effective against Escherichia coli, Bacillus subtilis, Staphylococcus aureus |
| Streptomyces species strain CNH365 | Anthracimycin | It has a potent activity against Bacillus anthracis, MRSA, and VRSA |
| Streptomyces formicae KY5 strain | Formicamycins A-L | It is effective against MRSA, vancomycin resistant Enterococci, but do not show any activity against gram-negative bacteria |
Table 2. Antibacterial compounds isolated from different actinomycetes strains and their spectrum of activity.
Macrolides
Macrolides are a well-known group of antibacterial compounds with a huge lactone ring comprising usually 12 atoms to 16 atoms, on which one or more deoxy sugar groups are attached by glycosidic bonds. These compounds are often utilized to combat respiratory tract diseases induced by fastidious gram-negative as well as gram-positive bacteria. In addition, they are used to treat other infections related to genital tract, skin, and soft tissues generated by mycoplasma species, some anaerobic bacteria, and several gram-positive and gram-negative bacteria.
Macrolides prevent the growth of bacteria by inhibiting protein synthesis through interruption of ribosome function. The details of such mechanism are explained by structure-basedapproaches including a high-resolution x-ray that shows the structure of ribosome-macrolide complexes. Yet, there are nearly 70 types of macrolide antibiotics used in clinical fields. They majorly include roxithromycin, mycinamycin, spiramycin, kitasamycin, erythromycin and its related substances, azithromycin, oleandomycin, rosaramicin, josamycin, tylosin, flurithromycin, clarithromycin, mirosamycin, dirithromycin, and rokitamycin [10].
In 2015, Lu et al. obtained two tylosin analogues, which are 16-membered macrolides, from Streptomyces ansochromogenes. These agents showed moderate effect on gram-positive bacteria including B. subtilis, Streptococcus pyogenes, S. aureus, Streptococcus pneumoniae, and B. cereus recording Minimal Inhibitory Concentration (MIC) ranging between 3.53 μg/ml and 58.5 μg/ml. It was found that tylosin analogues exhibited a greater bactericidal result in comparison to tylosin itself against S. pneumoniae.
Another study by Sawa et al. isolated a new macrolide, namely quadoctomycin, which is a 48-membered polyol macrolide, from a Streptomyces species, namely MM168-141F8.
This compound displayed a strong antibacterial activity against S. aureus. MIC estimates were recorded for five Staphylococcus aureus that were resistant to methicillin (MRSA), three S. aureus that were susceptible to methicillin (MSSA), and six strains of E. faecalis. The values were between 1 μg/ml and 2 μg/ ml. However, quadoctomycin have not recorded any antibacterial effect against gram-negative bacteria [11].
Aminoglycosides
Aminoglycosides are characterized by having amino sugars connected by glycosidic bonds to an aminocyclitol ring. They are a group of antibiotics with a broad-spectrum activity against different gram-positive and gram-negative bacteria. They are specifically effective against various members of Enterobacteriaceae family including Morganella spp., E. coli, Enterobacter cloacae, Serratia spp., Providencia spp., E. aerogenes, Proteus spp., Klebsiella pneumoniae, and K. oxytoca. Besides, aminoglycosides are potent against Francisella tularensis as well as Yersinia pestis, which cause tularemia, and plague, respectively. Also, aminoglycosides have good antibacterial effect against S. aureus, including strains that are intermediately resistant and resistant to vancomycin, as well as isolates resistant to methicillin.
Aminoglycosides act by inhibiting synthesis of proteins. They specifically bind to the A-site on 16S rRNA of the small ribosomal subunit. Despite that, different members of aminoglycosides bind different locations on the A-site, they all cause an alteration of its three-dimensional shape.
Consequently, misreading of the codon occurs on aminoacyl transfer RNA delivery, leading to mistranslation, allowing the link of a wrong amino acid to the polypeptide chain. Eventually, this leads to a release of this polypeptide chain causing an injury to the membrane of the cell [12]. Members of natural products isolated from Streptomyces species, including Streptomyces tenjimariensis, Streptomyces kanamyceticus, and Streptomyces spectabilis, end in-mycin (e.g., tobramycin, neomycin, streptomycin, kanamycin, and paromomycin). On the contrary, natural compounds isolated from Micromonospora species have them ending in-micin (e.g., gentamicin).
Tetracyclines
Tetracyclines are characterized by having a linear fused tetracycline nucleus, and 4-dimethylamino groups to which can be attached various functional groups. They are among broadspectrum antibacterial compounds active towards various gram-negative and gram-positive bacteria, obligate intracellular bacteria, protozoan parasites, and spirochetes. Tetracyclines exhibit their bactericidal activity by preventing formation of proteins. This occurs by not allowing the binding of aminoacyl-tRNA to its acceptor site (A-site) on the ribosome. The first members of the tetracycline class, chlortetracycline and oxytetracycline, were found near the end of 1940s. These compounds were produced by S. aureofaciens and Streptomyces rimosus, respectively. Different natural tetracyclines were discovered after that, for example demethylchlortetracycline isolated from S. aureofaciens, and tetracycline from S. rimosus, S. aureofaciens, and S. viridofaciens. Besides, semisynthetic products were brought into the clinical field including minocycline, methacycline, and doxycycline.
Ansamycins
Ansamycins are a class of antibiotics known for their aliphatic chain, attached to an aromatic moiety in two non-adjacent locations. Members of this class display potent antiviral, antibacterial, as well as anticancer activities. Rifampicin, a semisynthetic antibiotic, was the first clinically developed member in this class. Rifampicin and its derivatives showed strong antibacterial activity against mycobacteria, Neisseria, Listeria, Haemophilus influenzae, Borrelia burgdorferi, and Legionella pneumophila. Despite that hydrophobicity restricts their antibacterial activity against gram-negative bacteria [13]. Rifampicin and other members in the ansamycin family selectively block bacterial DNA transcription by inhibiting DNA-dependent RNA polymerase. Rifampicin is an important key component in the treatment of tuberculosis and is produced by Amycolatopsis mediterranei.
Angucyclines
Angucyclines constitute the biggest class of polycyclic aromatic polyketides synthesized by actinomycetes. They come from benzanthraquinone moiety. They are characterized by a four-ring frame of the aglycone group, which is joined in an angular way. This family has many biological activities including antibacterial, anticancer, antiviral, in addition to inhibition of enzyme activity and platelet aggregation. But due to the toxicity and solubility issues present in this family, none of their compounds has been incorporated into clinical development. Tetragomycin was the first compound identified in this class, which was obtained from the fermentation of Streptomyces rimosus. This antibiotic has a strong bactericidal effect against S. aureus, MRSA, and Streptococcus pyogenes. Benza-anthraquinones and angucylinones derivatives produced by Streptomyces species are well explored because of their broad biological activities [14].
Streptogramins
Streptogramins are a class of antibiotics with two distinct chemical groups. Streptogramins of group A (SA) are known for being polyunsaturated macro-lactones included in the polyketide family. On the other hand, streptogramins of group B (SB) are recognized as cyclic hexadepsipeptides belonging to the nonribosomal peptide antibacterial family. Each group alone acts as bacteriostat, limiting synthesis of proteins by reversibly binding to the large bacterial ribosomal component (50S). In contrast, when joined together, a synergistic impact is obtained with an elevated effect of 100 folds leading to the bactericidal activity of streptogramins. They have been used against a huge number of gram-positive bacteria including Enterococci and Staphylococcus aureus strains that are resistant to vancomycin, in addition t o few gram-negative bacteria.
Group A streptogramins are produced by Streptomyces virginiae, while Group B streptogramins are synthesized by different members of the genus Streptomyces.
Chloramphenicol
Chloramphenicol was first extracted from Streptomyces venezuelae isolated from soil in 1947. This compound, of the molecular formula C11H12C12N2O5, is an antibacterial agent with a bacteriostatic effect illustrated by inhibiting protein synthesis.
This activity targets a large number of organisms including spirochetes, gram-positive and gram-negative bacteria, and obligatory intracellular pathogens. Chloramphenicol competes with aminoacyl tRNA in attaching to the large ribosomal subunit, specifically on peptidyl-transferase site. As a result, a change in the ribosomal conformation is obtained, slowing down or inhibiting the association of the aminoacyl tRNA and thereby the transpeptidation reaction. Despite its bacteriostatic effect, chloramphenicol exhibits a toxic effect on mammalian cells, where it interacts with eukaryotic mitochondrial ribosomes. This may pose different health issues including depression of the bone marrow, displayed by thrombocytopenia, anemia, or leukocytopenia, thus requires further clinical investigations [15].
Beta lactams
β-Lactam antibacterial agents, possessing a β-lactam ring within their chemical formula, are the biggest family of antimicrobial substances, and the prominent agents currently in use in the clinical field. They include monobactams, penicillin, carbapenems, and cephalosporins. Beta lactams have potent bactericidal activity mostly against gram-negative bacteria, and to a limited degree, grampositive bacteria. These antibiotics inhibit Penicillin-Binding Proteins (PBPs), required for the transpeptidation of the peptidoglycan layer of the prokaryotic cell wall. However, the enzymes responsible for peptidoglycan cross-links hydrolysis remain active. The accumulation of peptidoglycan precursors leads to the stimulation of cell wall hydrolases, which further leads to the digestion of the intact peptidoglycan causing thus bacterial cell rupture. The gram-positive bacteria have a thicker peptidoglycan wall and are more cross-linked than gram-negative bacteria, this maintains the integrity of the former more [16].
Peptides
Out of the most fascinating classes of antibiotics, peptide antibiotics are the predominant group, mainly for inhibiting the growth of resistant bacterial strains. Among this group, there is a category called Non-Ribosomal Peptides (NRPs). NRPs have a non-peptide group or a non-proteinogenic amino acid attached to a peptide chain. Nearly 1,730 NRPs were identified in 2019. This category possesses a broad variety at the structural and compositional level. For this reason, they display a broad spectrum of chief biological effects. NRPs antibiotics can be grouped into three categories: Lipopeptides, glycopeptides, or glycolipopeptides, based on the type of the non-peptide moiety.
Lipopeptides have been discovered for more than 50 years. In general, they are composed of a hydrophilic cyclic peptide part linked to a fatty acid trail. The latter makes the placement into the phospholipid bilayer of the bacterial membrane easier. One of the characteristics of some lipopeptides is their dependence on calcium ions in order to perform their antimicrobial activity. Due to this, a discrete class of lipopeptide antibiotics have been created, which is the Calcium-Dependent lipopeptides (CDAs).
More than 40 CDAs have been discovered so far. Among which, daptomycin is a recently identified calcium-dependent antibiotic with various modes of action including the prevention of synthesis of the cell wall, perforation of the bacterial cell membrane, and alteration of the fluidity of the membrane. Glycopeptides and glycolipopeptides exhibit an antibacterial effect by preventing the biosynthesis of the bacterial cell wall. This may occur by preventing peptidoglycan biosynthesis, and specifically the trans-glycosylation procedure, or by interacting with the end moieties, namely D-alanyl-D-alanine, of the NAM/NAG-peptides [17].
Sulfonamides
Sulfonamide antibiotics, with SO2-NH2 moiety, are synthesized by Streptomyces species. These antibiotics have the same structure as Para-Aminobenzoic Acid (PABA), which makes them competitive antagonists in bacterial cells. In general, microorganisms possess an enzyme, namely dihydropteroate synthetase that utilizes PABA to create dihydrofolic acid, which is a precursor of folic acid. Microbes use folic acid to synthesize purines and pyrimidines. Sulfonamides block the conversion of PABA into dihydropholic acid, affecting folates synthesis, thus inhibiting nucleic acid synthesis. Moreover, sulfonamide antibiotics get incorporated into the precursors, generating a reactive pseudometabolite with an antibacterial activity. Sulfa drugs were the first drugs identified against bacterial septicemias. Besides, they were reported effective in treating bacterial infections including tissue infections caused by Streptococcus. Eukaryotic cells are not affected by sulfonamides, because they already absorb folic acid, resulting in a broad therapeutic index.
A source of antifungal agents
Antifungal Peptides (AFPs) synthesized by actinomycetes exhibit a broad-spectrum antifungal activity by various mechanisms. One way is through inhibiting the formation of the cell wall by inactivating (1-3)-β-D-glucan synthase and Chitin Synthase (CHS). This disrupts the normal cell shape and reduces the cell capability of osmotic pressure regulation. Another way is showing activity against cell membranes and different intracellular compounds including nucleotides (DNA and RNA), proteins, and mitochondrial membrane. Table 3 summarizes the bioactive compounds including those used as antifungal agents [18]. Kribellosides A-D, which are four newly discovered inhibitors of Cet1p (RNA 5’-triphosphatase), were taken from a novel actinomycete, namely Kribella MI481-42F6, located in the soil of Nerima-ku, Japan. These new alkyl glyceryl ether products inactivated, in vitro, the enzyme RNA 5’ triphosphatase present in Saccharomyces cerevisiae with an IC50 of 5 μM-8 μM and displayed antifungal activity against S. cerevisiae with MIC between 3.12 μg/ml to 100 μg/ml.
Six newly identified cyclic octa depsipetitdes, namely enduspeptides A-F, were taken from a Streptomyces strain collected from soil of a coal mine, 20 cm deep from the surface of ground, in Nanchang, Jiangxi, Republic of China. The products were tested for their activity against fungi, and it turned out the most potent activities were recorded for enduspeptides A, B, and C, where they recorded 5.33 μg/ml, 1.72 μg/ml, and 8.13 μg/ml IC50 values, respectively against C. glabrata. Mohangamides A and B were isolated from an aquatic Streptomyces species SNM55 collected from Mohang mud flat obtained from Korea. These are peptides with 14 amino acids possessing two unfamiliar acyl chains. The antifungal activities of these products were assessed against Isocitrate Lyase (ICL) found in C. albicans. This enzyme has a primary function in the glyoxylate cycle and permits microbes to live and proliferate on ethanol, acetate, or fatty acids in their habitats. Mohangamides A and B showed an inhibiting activity against ICL recording IC50 number of 4.4 μM and 20.5 μM, respectively. But the antifungal activity of these compounds decreased significantly when the fungi were given glucose. However, when C. albicans was allowed to proliferate on 2% sodium acetate, mohangamide A inhibited its growth.
Nikkomycins and polyoxins are two groups of antifungal nucleotides competitively preventing the activity of chitin synthase found in fungi. Polyoxins are metabolites isolated in the 1960s from Streptomyces cacaoi of subspecies asoensis with an antifungal effect against fungi pathogenic to plants (e.g., Pricularia oryzae, and Alternaria kikuchiana). On the other hand, nikkomycins (nikkomycin Z) show a stronger antifungal activity against Candida albicans compared to polyoxins. Nikkomycin compounds isolated in the 1970s from S. tendae exhibit a potent activity against Botrytis cinerea and Rhizopus carcinans. Nikkomycin X and Z are included within the peptidyl nucleoside class of antibiotics and are synthesized by S. ansochromogenes. These two compounds are very similar in structure, but nikkomycin Z shows a stronger antifungal activity than nikkomycin X. Due to this, Liao et al. manipulated Streptomyces genes to obtain selective production of nikkomycin Z [19].
A source of insecticidal agents
About 60% of the novel herbicides and insecticides are produced by Streptomyces species. These compounds are of high interest because of their high specificity and ability to degrade, thus their safe effect on the environment. However, acquiring knowledge concerning the mode of action of these biological compounds is needed to develop commercial products of actinomycetes origin with a prolonged shelf-life. Table 3 summarizes the bioactive compounds including those used as insecticidal agents. Avermectins are 16-membered, naturally occurring or semi-synthetic macrocyclic lactone derivatives that are synthesized by the soil-swelling Streptomyces avermitilis during its fermentation. It has both insecticidal and anthelmintic activities. It is utilized to control mite pests and insects in vegetable, fruit, agronomic, and ornamental crops.
Avermectin is mostly used to eliminate fire ants. It is also effective in controlling lungworm, intestinal nematode, tick, and lice. The therapeutic effect of avermectins is exerted by binding the latter to glutamate-gated chloride channels, causing flaccid paralysis and then killing of the parasite. Initially, avermectins were thought to act against parasites by increasing the production of GABA. However, the major mode of action was found by binding specifically to glutamate-gated chloride channels in muscle and neural cells.
Ivermectin is an anti-helminthic dehydro-derivative of avermectin secreted by Streptomyces avermitilis. It was first developed at the end of 1970s and was considered the initial endectocide available. Ivermectin was recognized as a new group of antiparasitic compounds possessing a potent wide effect against external and internal arthropods and nematodes. Nowadays, ivermectin is efficient against different parasites including gastrointestinal roundworms, mites, hornflies, lice, and lungworms. Also, it was recorded efficient against ticks, such as the ixodid tick, Rhipicephalus (Boophilus) microplus, which is the main cattle parasite in subtropics and tropics, thus causing massive economic damage. The therapeutic effect of ivermectin is exerted by attaching to glutamate-gated chloride channels present in insects and nematodes, leading to chloride ion entry and consequently, the cell hyperpolarization resulting in its dysfunction. It is noteworthy that ivermectin was used by many countries as a first-line treatment option for COVID-19. Ivermectin was also tested for decreasing secondary outcomes of COVID-19, deaths, and in chemoprophylaxis, among individuals with COVID-19 infection [20].
Spinosyns are broad range of insecticides. In laboratory bioassays, spinosad, a natural cocktail including the main compound spinosyn A and the minor compound spinosyn D, has shown efficiency against permethrin-resistant lice infecting the head and permethrin-susceptible lice infecting the body. Currently, Para PRO LLC (Carmel, IN, USA) is developing a creme rinse product called natrova that contains spinosad as the active ingredient for treating head lice. Besides, spinosyns exhibit potent effect against major external livestock parasites and are utilized against insects of sanitary interest. Elector, which contains spinosad as its active component, was recently licensed in the United States for the management of hornflies and feeding lice on livestock. Spinosyns are generated from the fermentation process of Saccharopolyspora spinosa inhabiting the soil. Their basic structure is a polyketide-derived tetracyclic macrolide that possesses two attached saccharides, an amino sugar (D-forosamine), and a neutral sugar (L-rhamnose). Within this family, members are different in the degree of O- and N-methylation on the saccharides, or C-methylation on the polyketide nucleus. Spinosyns are of much importance because they have a low impact on the environment and are highly specific to the target insects. Besides, they have a unique mode of action preventing target species from resistance. As such they function as allosteric activators of nicotinic acetylcholine receptors. When compared with other insecticides, the spinosyns are more selective toward their target insects and have less activity against avian and aquatic animals, mammals, as well as beneficial predators. The unique mode of action of spinosyns, their broad insecticidal spectrum, and low environmental impact make them important products for the new integrated pest control strategies. The insecticidal potential of actinomycetes is not only designated by the bioactive products synthesis, but also by the release of chitinase enzyme, which digests the chitin surface of the insect, thereby permitting the toxic deadly compounds to penetrate the insect body. Chitin is an insoluble linear β-1, 4-linked polymer of N-acetylglucosamine that is degraded by chitinase into chitobiose, N-acetylglucosamine, and glucosamine. These end products can be used by actinomycetes to synthesis also chitinase. Nearly 90%-99% microbes producing chitinase enzymes are actinomycetes, and specifically Streptomyces species including Streptomyces lividans, S. griceus, S. aureofaciens, S. virdificans, S. thermoviolaceus, S. halstedii, S. plicatus, and S. diasitapiticus. Non- Streptomyces and Streptomyces species were collected and checked for their potential to release chitinase enzyme on Colloidal Chitin Agar (CCA). Results showed that Streptomyces sp. had a greater potential to produce chitinase. This is because Streptomyces exhibit multiple chitinase genes.
A source of anticancer agents
Actinomycetes are considered as the main reservoir of different antitumor compounds. These compounds belong to wide range of classes including macrolides, anthracyclines, enediynes, isoprenoides, indolocarbazoles, and non-ribosomal peptides. Their antitumor activity is exhibited by triggering apoptosis through different mechanisms. Table 3 summarizes the bioactive compounds including those used as anticancer agents. The first natural product utilized in cancer treatment was actinomycin D produced by S. antibioticus. This metabolite binds to the transcription initiation complex, thereby inhibiting the elongation of RNA by RNA-polymerase. Actinomycin D is actively used until now for treating Wilms tumors in kids, but due to its adverse side impacts, its usage has been limited.
Another active compound, Doxorubicin (DOX), is an anthracycline preventing the proliferation of cancer cells by intercalating to DNA and preventing topoisomerase II from functioning. DOX was first isolated from Streptomyces peucetius in the 1960s. It is being used for treating different cancers including lung, thyroid, breast, ovarian, gastric, non-Hodgkin’s and Hodgkin’s lymphoma, sarcoma, multiple myeloma, and pediatric cancers. However, due to the serious dose-dependent toxicity to the cardiac muscle, such as congestive heart failure and cardiomyopathy, its therapeutic value has been reduced. This toxicity is thought to be due to redox cycling of DOX in the mitochondria to produce superoxide anion or a different Reactive Oxygen Species (ROS) and thus causing oxidative stress.
Bleomycin is another anticancer agent isolated from Streptomyces verticillus. It is used to treat testicular tumors, squamous cell carcinoma of the neck and head, and Hodgkin’s lymphoma. Bleomycin’s antitumor activity is represented by its ability to degrade DNA. It prevents thymidine from incorporating into the DNA. In-vitro studies showed that this antitumor agent induces DNA degradation by depending on metal ions like iron, and oxygen. Bleomycin chelates iron, leading to the generation of a pseudo-enzyme that creates superoxide and hydroxide free radicals, which in turn cleaves the DNA. Other modes of action included in bleomycin-induced cytotoxicity are oxidative RNA degradation, lipid peroxidation, and topoisomerase II enzyme inhibition. Bleomycin is approved by FDA for cancer treatment in 1973.
Furthermore, Mitomycin C (MMC) is a chemotherapeutic agent having antiproliferative activities. It is potent against different solid tumors, including bladder, breast, lung, and gastrointestinal tumors (upper gastrointestinal, anal). MMC is isolated from the Streptomyces caespitosus fermentation broth. It possesses unsaturated ring structures and is categorized as a cell cycle, non-selective alkylating product. The MMC’s active metabolite makes cross-linking with DNA and thereby inhibiting DNA synthesis. Besides, because the activities of MMC are not only relying on the stage of the cell cycle, it can function in inhibiting protein synthesis, and mitosis. This prevents growth of cells and decreases the production of myofibroblasts from progenitor cells. However, despite its strong antitumor activity, MMC use in clinical treatment is limited because of its toxicity.
In addition, calicheamicin is a group of strong enediyne antitumor compounds taken from the Micromonospora echinospora fermentation broth. It is considered one of the most important Antibody-Drug Conjugates (ADCs). Specifically, it has been connected to an antibody that distinguishes CD33 expressed by tumor cells to treat acute myeloid leukemia. Calicheamicin cut DNA in a specific site in a double-stranded manner. Once bound, it gets reduced by cellular thiols. The produced compound rearranges itself so that it creates a 1,4- dehydrobenzene diradical eliminating hydrogen atoms. Calicheamicin is nearly 4000 times more potent than doxorubicin, frequently leading to the destruction of DNA of healthy cells, and thus should not be utilized alone as a treatment.
| Actinomycetes strain | Bioactive compound | Biological activity | Spectrum of activity |
|---|---|---|---|
| Kribella MI481-42F6 | Kribellosides A-D | Antifungal | It is effective against S. cerevisiae |
| Streptomyces sp. | Enduspeptides A-F | Antifungal | It is active against C. glabrata |
| Streptomyces species SNM55 | Mohangamides A and B | Antifungal | It is potent against C. albicans |
| Streptomyces ansochromogenes | Nikkomycins | Antifungal | It is effective against C. albicans |
| Streptomyces avermitilis | Avermectins | Insecticidal and anthelmintic | It eliminates mite pests, fire ants, lungworm, intestinal nematode, tick, and lice |
| Streptomyces avermitilis | Ivermectin | Antiparasitic | It is efficient against arthropods, nematodes, gastrointestinal roundworms, ticks, mites, hornflies, lice and lungworm |
| Saccharopolyspora spinosa | Spinosyns | Insecticidal | It is effective against major external livestock parasites |
| Streptomyces antibioticus | Actinomycin D | Antitumor | Treat Wilms tumors in kids |
| Streptomyces peucetius | Doxorubicin | Antitumor | Treating different cancers including lung, thyroid, breast, ovarian, gastric, non-Hodgkin’s and Hodgkin’s lymphoma, sarcoma, multiple myeloma and pediatric cancers |
| Streptomyces verticillus | Bleomycin | Antitumor | It is used to treat testicular tumors, squamous cell carcinoma of the neck and head and Hodgkin’s lymphoma |
| Streptomyces caespitosus | Mitomycin C | Antitumor | It is potent against different solid tumors, including bladder, breast, lung and gastrointestinal tumors |
| Micromonospora echinospora | Calicheamicin | Antitumor | It is used to treat acute myeloid leukemia |
Table 3. Bioactive compounds isolated from different actinomycetes strains and their spectrum of activity.
Actinomycetes are gram-positive bacteria found in different ecosystems around the world, primarily the soil. They have a complex structure characterized by thread-like mycelia. Actinomycetes are widely diverse and categorized according to morphological, chemotaxonomic, and molecular criteria. They are one of the most important prokaryotes on the biotechnological level and constitute the major reservoir of key secondary metabolites used in clinics. The emergence of antibiotic resistance, various fungal and parasitic infections, and tumor development establish the need for urgent solutions. Researchers are directed toward natural sources to discover novel secondary metabolites. Actinomycetes constitute the largest hope to get more medically and agriculturally useful elements that might provide direct drugs or structurally modified drug derivatives.
By introducing marine and terrestrial samples into new enrichment media and whole-cell modern screening methods, more actinomycetes secondary metabolites can be isolated. Besides, genomes of actinomycetes can be sequenced to give insights to provide new agents. Moreover, old actinomycetes products can be chemically modified in their original structure given by nature to provide an improvement in their effects, thereby making them effective against different microorganisms. This is possible due to the advanced research techniques that permit the combination between biology and chemistry. In this review, we have gathered an updated overview about the characteristics of actinomycetes and shed light on the main metabolites they secrete and their different biological activities. These metabolites could serve as potential and promising therapeutic agents for many microbial infections and could replace other synthetic treatments with serious side effects.
None.
Rayan Zahr: Conceptualization, data curation, writing and editing-original draft. Sarah Zahr: Writing and editing-original draft. Rana El Hajj: Validation, writing-review, editing and supervision. Mahmoud Khalil: Principal investigator and project Supervision. All authors have read and approved the final version of the manuscript.
None.
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
Citation: Zahr R, Zahr S, Hajj RE, et al.. Actinomycetes, Promising Therapeutic Agents: Characteristics and Active Metabolites. J Biol Todays World, 2023,12(1), 1-8.
Received: 07-Jun-2022, Manuscript No. JBTW-22-66081; Editor assigned: 10-Jun-2022, Pre QC No. JBTW-22-66081(PQ); Reviewed: 24-Jun-2022, QC No. JBTW-22-66081; Revised: 07-Jan-2023, Manuscript No. JBTW-22-66081(R); Published: 17-Jan-2023, DOI: 10.35248/2322-3308.12.1.002
Copyright: © 2023 Zahr R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.