Research Article - (2021) Volume 10, Issue 3
Introduction: Guadeloupe, a French West Indies island, has been fiercely affected by two large waves of COVID. Therapeutic approach was different between the two waves in the Intensive Care Unit (ICU). We aimed to compare the two different periods in terms of characteristics and outcomes and to evaluate risk factors associated with 60-day mortality in our overall cohort.
Methods: All consecutive patients with laboratory confirmed COVID-19 pneumonia and requiring oxygen support admitted in our ICU unit of University Hospital of Guadeloupe were prospectively included. Patients were treated during the first wave with a combination of Hydroxychloroquine and Azithromycin and during the second wave with dexamethasone and reinforced anticoagulation.
Results: In our cohort, 187 patients were included, 31 during the first one and 156 during the second. Patients were mostly male (69%) with a median age of 64 years old. Patients tend to be younger during the second wave and body mass index was higher (respectively 31 vs. 27 Kg/m2, p=0.01). Overall mortality at Day 60 was high (45%) and not different between the two waves. Among patients under mechanical ventilation risk factors associated with death in a multivariate analysis were a high number of comorbidities, a high level of SOFA score and the delay of Invasive Mechanical Ventilation (IMV) onset after admission in ICU (OR=1.6, 95% CI 1.2-2.4).
Conclusion: Although therapeutics approach evolves, COVID-19 severe pneumonia is still associated with a high mortality rate in ICU.
COVID 19 • Epidemic • Acute respiratory distress syndrome • Population • Pulmonary fibrosis • Epidemiology • Ventilator associated pneumonia
Coronavirus disease 2019 (COVID-19) pandemic has been, since it was first described, a field of investigation for physicians worldwide [1]. Since March 2020, Guadeloupe, a French Caribbean island has been affected by two large waves of COVID-19 cases. During the first wave, (March to May 2020), the disease was poorly understood and had no effective treatment. Since then, large studies have been published and have identified prognostic factors related to the host and the level of organ dysfunction [2]. Since the beginning of the outbreak, specific SARS CoV-2 therapy has been intensively searched [2,3].
During the first wave, we used hydroxychloroquine with azythromycin, as no therapy was clearly identified at that time. Standard of care has greatly evolved since and now includes steroids due to preliminary data suggesting reduced 30-day mortality [2-5]. COVID-19 has also been shown to be associated with coagulopathy and a high risk of thrombosis, and several sets of guidelines suggest keeping up keeping a sufficient level of anticoagulation among SARS CoV-2 infected patients [6-10]. New drugs are still under evaluation 6 though there is no great consensus on their use at this time. Currently, whether any therapeutic approaches have an impact on morbi-mortality remains unclear [11]. We report here our cohort of patients admitted to an Intensive Care Unit (ICU) with COVID-19 laboratory confirmed severe pneumonia during the two main waves in 2020. We compared the two groups in terms of clinical characteristics and outcomes. We also evaluated risk factors associated with 60-day mortality in these critically ill patients.
Population selection and study design
COROCARA is a single center prospective cohort study conducted in the ICU of the University Hospital in Guadeloupe. All patients over 18 years of age with COVID-19 pneumonia and hospitalized in the ICU during the two COVID-19 outbreaks from March-May (first wave) and August-October (second wave) were included in the study. COROCARA received approval from the ethics committee of the University Hospital in Guadeloupe. Laboratory confirmation was defined as a positive result by real-time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) assay from either nasopharyngeal swabs or lower respiratory tract aspirates. All included patients had a laboratory confirmed diagnosis.
Data collection
All clinical and biological data were collected within the first twentyfour hours after ICU admission. For each patient we collected the following clinical data: age, sex, Body Mass Index (BMI), comorbidities, immunodeficiency, treatment received, the Simplified Acute Physiology Score (SAPS) II score, the Sequential Organ Failure Assessment (SOFA) score, date of first symptoms, dates of hospital and ICU admission, support devices (oxygen mask, high flow nasal cannula, or Non-Invasive Ventilation (NIV)) at admission, or IMV [12,13]. Routine laboratory data included blood cell count, electrolytes dosages, liver enzymes, blood cultures, blood gas, Creatine PhosphoKinase (CPK), D-dimeres and troponin. When possible, each patient underwent a chest computed tomography before admission.
Syndrome and outcome
ARDS was graded based on the Berlin Definition for patients undergoing mechanical ventilation (invasive or non-invasive) [14]. To be comparable to other previously published studies, ARDS was only graded in patients receiving mechanical ventilation on ICU day 1. In this study, ICU-complications and organ dysfunction included acute kidney failure requiring renal replacement therapy, pulmonary embolism (proven by pulmonary CT angiography), ventilator-associated pneumonia, and cardiac arrest. Clinical suspicion of ventilator-associated pneumonia was confirmed before antibiotics either by blind protected specimen brush growing ≥ 103 cfu/ml, or endotracheal aspirates growing ≥ 106 cfu/ml. Patient outcomes was recorded at the ICU or at hospital discharge. A favorable outcome was defined as a patient who was alive at day 60 after admission.
Treatment
Standard of care was different during the first and second wave, due to an increased availability of data and results in the literature over time and an improving understanding of the disease. Hydroxychloroquine was initially used during the first wave in combination with azithromycin in our center, even though its use was debated at the time. Dexamethasone (steroids) was systematically used for severe patients requiring oxygen during the second wave, and at the discretion of the physician during the first. In the second wave, anticoagulation was reinforced, systematic screening for pulmonary embolism was performed, and NIV and high flow oxygen were used. The use of mechanical ventilation was at the discretion of the physician.
Statistical analysis
All analyses were performed using R 4.0.4 [15]. Data are reported as median (interquartile range) or number (percentage). The baseline data are reported from the twenty-four hours period after ICU admission. No sample size calculations were performed. Univariate characteristics of the two cohorts (first and second wave) were compared using chi-square or Fisher's exact tests for categorical variables and using Student's t-test or Wilcoxon's rank-sum test for continuous variables. Kaplan-Meier overall survival curves up to day 60 were computed separately for first wave and second wave patients, and in patients with delayed mechanical ventilation (>4 days after ICU admission) vs. those mechanically ventilated starting in the first four days after treatment administration. No imputation was performed for missing values. We furthermore ran a multivariate analysis to asses risk factors for death in patients requiring IMV. The final set of variables to be included in the multivariate logistic model were chosen on the basis of pathophysiological interest and the requirement p<0.2. We performed backward selection on the model, stopping when the Akaike Information Criterion (AIC) reached its minimum.
Patients enrolled
Patient characteristics and their day 1 vital status are described in Table 1. Patients were majority male (n=129, 69%) with median age 64 years (54-71). Within twenty-four first hours, the median SAPS II was 34(24-46) and the median SOFA was 4(3-8). Patient characteristics were strikingly similar between the two waves for most of evaluated variables. Median respiratory rate and median Body-Mass Index were higher during the second wave (respectively, 34 vs. 31/min, p=0.03, and 30.9 vs. 27.2 kg/m2, p=0.01). Few patients had a bacterial coinfection at admission (n=10, 6%).
| All N=187 |
First wave n=31 |
Second wave n=156 |
p-value | |
|---|---|---|---|---|
| Age (years) | 64.0(54.5, 71.0) | 68.0(59.5, 75.5) | 64.0(53.0, 71.0) | 0.06 |
| Male | 129(69.0%) | 22(71.0%) | 107(68.6%) | 0.96 |
| Age catégories (years) | 0.39 | |||
| (18-50) | 34(18.2%) | 3(9.68%) | 31(19.9%) | |
| (50-60) | 25(13.4%) | 5(16.1%) | 20(12.8%) | |
| (60-70) | 68(36.4%) | 10(32.3%) | 58(37.2%) | |
| (70) | 60(32.1%) | 13(41.9%) | 47(30.1%) | 0.003 |
| Body Mass Index (kg/m2) | 29.4(26.1, 34.2) | 27.2(24.7, 28.9) | 30.9(26.5, 34.6) | 0.01 |
| Time 1st symptoms-ICU. admission (days) | 8.00(5.50, 11.0) | 8.00(5.50, 12.0) | 7.50(5.75, 11.0) | 0.49 |
| Comorbidity | ||||
| Hypertension n(%) | 118(63.1%) | 20(64.5%) | 98(62.8%) | 1 |
| Diabetes n(%) | 98(52.4%) | 14(45.2%) | 84(53.8%) | 0.49 |
| Other Cardiac disease n(%) | 20(10.7%) | 3(9.68%) | 17(10.9%) | 1 |
| Chronic kidney disease n(%) | 25(13.4%) | 1(3.23%) | 24(15.4%) | 0.08 |
| Malignancy | 12(6.42%) | 1(3.23%) | 11(7.05%) | 0.69 |
| Number of comorbidities | 2.00(1.00, 2.00) | 1.00(1.00, 2.00) | 2.00(1.00, 3.00) | 0.2 |
| Previous treatment | ||||
| RAAS inhibitors n(%) | 59(31.6%) | 12(38.7%) | 47(30.1%) | 0.47 |
| Metformin n(%) | 42(22.5%) | 9(29.0%) | 33(21.2%) | 0.47 |
| Clinical data at Day 1 | ||||
| Temperature (°C) | 38.0(37.1, 38.5) | 38.3(37.8, 39.2) | 37.8(37.0, 38.3) | 0.04 |
| Respiratory rate (/min) | 34.0(28.0, 40.0) | 31.0(25.0, 35.5) | 34.0(29.0, 40.0) | 0.04 |
| Diarrhea n(%) | 37(20.2%) | 6(19.4%) | 31(20.4%) | 1 |
| Confusion n(%) | 16(8.74%) | 2(6.45%) | 14(9.21%) | 1 |
| PaO2/FiO2 at D1 (n= 68/72) | 120(90.0, 180) | 168(116, 215) | 110(88.5, 160) | 0.04 |
| SAPS II | 34.0(24.0, 46.0) | 32.0(24.0, 47.0) | 34.0(24.0, 45.2) | 0.87 |
| SOFA | 4.00(3.00, 8.00) | 4.00(3.00, 8.00) | 4.50(3.00, 8.00) | 0.89 |
| Biological data at Day 1 | ||||
| Hemoglobin (g/L) | 12.4(11.0, 13.8) | 12.8(11.2, 14.4) | 12.4(10.9, 13.6) | 0.24 |
| Platelets (G/L) | 222(168, 294) | 186(146, 238) | 230(177, 294) | 0.02 |
| Leucocytes (G/L) | 9.20(6.60, 11.6) | 6.90(5.85, 8.85) | 9.60(7.00, 11.8) | <0.001 |
| Lymphocytes (G/L) | 0.95(0.62, 1.27) | 1.10(0.59, 1.23) | 0.91(0.62, 1.27) | 0.63 |
| DDimers (µg/mL) n=30 | 1.47(0.96, 3.33) | 1.88(1.06, 3.86) | 1.42(0.92, 2.66) | 0.13 |
| AST UI/L | 57.0(38.8, 86.5) | 65.0(52.0, 101) | 56.0(37.0, 86.0) | 0.15 |
| ALT UI/L | 40.0(25.0, 63.0) | 43.0(34.0, 64.0) | 39.0(25.0, 62.5) | 0.39 |
| CRP (mg/L) | 162(84.8, 267) | 165(100, 261) | 161(84.0, 273) | 0.97 |
| Creatinin (µmol/L) | 95.0(72.2, 151) | 88.0(70.0, 110) | 100(73.0, 158) | 0.13 |
| CPK (UI/L) | 332(154, 960) | 548(168, 1368) | 293(152, 894) | 0.21 |
| LDH (UI/L) | 560(465, 693) | 591(464, 682) | 557(468, 693) | 0.90 |
| Troponin (ng/mL) | 0.02(0.01, 0.06) | 0.02(0.01, 0.06) | 0.03(0.01, 0.06) | 0.62 |
| % infiltrate on chest computed tomography | 0.23 | |||
| = 25% | 14(8.14%) | 1(3.57%) | 13(9.03%) | |
| 26%-50% | 58(33.7%) | 6(21.4%) | 52(36.1%) | |
| 51%-75% | 55(32.0%) | 13(46.4%) | 42(29.2%) | |
| >75% | 45(26.2%) | 8(28.6%) | 37(25.7%) |
Note: Results are number n (percentage) of patients for categorical variables and median (q1-q3) for continuous variables. P-values were obtained using chi-square or Fisher's exact tests for categorical variables and using Student's t-test or Wilcoxon's rank-sum test for continuous variables.
First wave: March-May 2020; Second wave: August-October 2020; RAAS: Renin Angiotensin System; BMI: Body Mass Index; SAPSII: Simplified Acute Physiology Score; SOFA: Sepsis-related Organ Failure Assessment; CRP: C-Reactive-Protein; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; LDH: Lactate DesHydrogenase; CPK: Craatin PhosphoKinase. Day 1 is the first day of admission in Intensive Care Unit.
Table 1: Clinical, biological and radiological features of patients the day of ICU admission.
Ventilatory support, adjunctive therapies, and ARDS severity comparison
Within the first twenty-four hours, first wave patients more often received Invasive Mechanical Ventilation (IMV) (45% vs. 39%). In the second wave, patients often received high flow oxygen or NIV (51% vs. 0%) (Table 2). However, there was no significant difference regarding requiring IMV at some point during ICU hospitalization between the two periods (71% vs. 63% of patients (p=0.47)). Mechanical ventilation-associated therapies used for ARDS management including prone positioning (n=85, 71% in total) and the use of a neuromuscular blockade (n=106, 89% in total), were not significantly different between the two waves (Table 2).
| All N=187 |
First wave n=31 |
Second wave n=156 |
p-value | |
|---|---|---|---|---|
| Oxygen administration within 24 first hours | <0.001 | |||
| Standard oxygen therapy | 35(19.00%) | 17(54.8%) | 18(11.62%) | |
| Mechanical ventilation | 72(40.90%) | 14(45.2%) | 58(38.90%) | |
| High flow oxygen | 65(34.90%) | 0(0.00%) | 65(41.90%) | |
| Non-invasive ventilation | 14(7.95%) | 0(0.00%) | 14(9.40%) | |
| ARDS classification | 0.13 | |||
| Mild | 14(17.3%) | 6(33.3%) | 8(12.7%) | |
| Moderate | 36(44.4%) | 7(38.9%) | 29(46.0%) | |
| Severe | 31(38.3%) | 5(27.8%) | 26(41.3%) | |
| Pulmonary embolism | 25(13.7%) | 7(22.6%) | 18(11.8%) | 0.14 |
| Mechanical ventilation required during ICU | 119(64.0%) | 22(71.0%) | 97(62.6%) | 0.47 |
| Complications and management (n=119) | ||||
| Time between hospital admission and mechanical ventilation(days) | 2.00(1.00, 4.00) | 1.50(0.25, 2.00) | 2.00(1.00, 4.00) | 0.007 |
| Time between ICU admission and mechanical ventilation(days) | 0.00(0.00, 1.00) | 0.00(0.00, 1.00) | 0.00(0.00, 2.00) | 0.61 |
| Renal replacement therapy | 29(25.20%) | 8(36.40%) | 21(22.60%) | 0.29 |
| Continuous neuromuscular blockers | 106(89.10%) | 21(95.50%) | 85(87.60%) | 0.46 |
| Prone position | 85(71.40%) | 16(72.70%) | 69(71.10%) | 1 |
| ECMO | 8(6.78%) | 2(9.09%) | 6(6.25%) | 0.64 |
| Norepinephrine | 86(73.50%) | 21(95.50%) | 65(68.40%) | 0.02 |
| Infectious complications | ||||
| Ventilator associated pneumonia | 56(50.5%) | 14(63.6%) | 42(47.2%) | 0.25 |
| Bacteriemia | 39(37.1%) | 14(63.6%) | 25(30.1%) | 0.008 |
| Outcome | ||||
| D60 Mortality | 83(44.6%) | 13(41.9%) | 70(45.2%) | 0.92 |
| Length of stay in ICU(days) | 7.00(4.00,15.2) | 14.0(6.50,28.0) | 6.00(4.00,13.0) | 0.002 |
| Length of mechanical ventilation(n=119) | 8.00(5.00,17.0) | 20.0(12.8,23.2) | 7.00(4.00,15.0) | <0.001 |
Note: Results are median and (IQR 25-75) for continuous variables and number n(%) for categorical variables, p values were obtained using chi-square or Fisher's exact tests for categorical variables and using Student's t-test or Wilcoxon's rank-sum test for continuous variables First wave: March-May 2020; Second wave: August-October 2020; ARDS: Acute Respiratory Distress Syndrome; ICU: Intensive Care Unit; ECMO: Extracorporeal Membrane Oxygenation.
Table 2: ICU management, complications and outcome between first and second wave (N=187).
ICU complications and organ support in patients requiring mechanical ventilation
Ventilator-associated pneumonia was diagnosed in 50% of patients who received IMV while 25% had acute kidney failure requiring renal replacement therapy. No statistical differences were observed between the two waves for these two variables. Median length of IMV was longer among patients during the first wave rather than in patients during the second wave (respectively 20 days vs. 7, p<0.001).
Patient outcomes and predictors of 60-day mortality
Results of the univariate and multivariate analysis are reported in Tables 1-3. Non-Survivors within 60 days were older, had more comorbidities at admission than survivors (OR 95% CI: 1.65 (1.1; 2.7), p=0.04) and had much higher renal and hemodynamic SOFA component scores. Time to mechanical ventilation was also associated with death within 60 days with an OR of 1.6 (95% CI 1.2-2.4).
| OR (95%CI) | p-value | |
|---|---|---|
| Age | 1.03(1.0,1.1) | 0.1 |
| SOFA score | 1.34(1.15, 1.61) | <0.001 |
| Number of comorbidities | 1.63(1.1,2.6) | 0.04 |
| Time to mechanical ventilation (days) after ICU admission | 1.64(1.2, 2.4) | 0.005 |
Results from univariable analyses are presented in the appendix. OR and 95%CI: Odds Ratios and 95% Confidence Interval were calculated from the multivariable model after deletion of patients with missing data (N=9), 76(64%) patients died within 2 months, SOFA: Sequential Organ Failure Assessment; VAP: Ventilator Acquired Pneumonia; ICU: Intensive Care Unit
Table 3: Multivariable logistic recession analyses of factors associated with 60 days mortality for patients who required Mechanical ventilation during ICU (N=109).
Kaplan-Meier survival curves are presented in Figure 1. There were no statistical differences in mortality rates between the two periods. During the second wave, high flow oxygen and NIV were often used as first-step therapy (51% of patients within the first twenty-four hours), thus delaying IMV for 25% of the patients (39/156) (Table 4).
| All N=34 |
NIV >4days n=16 |
MV after 4 days n=18 |
OR (95%CI) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 64.0 (56.5, 67.0) | 61.5 (49.5, 67.0) | 64.0 (61.5, 71.0) | 1.08 (1.00, 1.1) | 0.05 |
| Body Mass Index (kg/m2) | 28.2 (25.1, 33.0) | 28.1 (26.4, 33.0) | 28.2 (23.8, 31.0) | 0.98 (0.89,1.0) | 0.73 |
| Time 1st symptoms-ICU admission (days) | 10.0 (6.25, 14.0) | 10.0 (7.25, 13.0) | 11.0 (6.25, 15.0) | 1.04 (0.92,1.1) | 0.54 |
| Comorbidity | |||||
| Hypertension n(%) | 21 (61.8%) | 8 (50.0%) | 13 (72.2%) | 2.50 (0.60, 11.0) | 0.21 |
| Diabetes n(%) | 17 (50.0%) | 6 (37.5%) | 11 (61.1%) | 2.52 (0.63, 11.0) | 0.19 |
| Number of comorbidities | 3.00 (1.25, 4.00) | 2.00 (1.00, 3.0) | 3.50 (2.25, 5.0) | 1.91 (1.12, 3.2) | 0.01 |
| Clinical data at Day 1 | |||||
| Fever (°C) | 37.6 (37.0, 38.0) | 37.6 (37.0, 38.0) | 37.2 (37.0, 38.0) | 0.74 (0.32,1.7) | 0.48 |
| Respiratory rate (/min.) n=36 | 34.0 (5.93) | 34.6 (6.23) | 33.4 (5.78) | 0.97 (0.86,1.0) | 0.57 |
| SAPS II | 30.0 (24.0, 37.0) | 25.0 (22.0, 30.0) | 37.0 (26.0, 46.0) | 1.15 (1.03,1.2) | 0.01 |
| SOFA | 3.00 (2.00,5.0) | 3.00 (2.00,4.0) | 5.00 (3.00, 8.0) | 1.51 (1.03, 2.2) | 0.03 |
Note: Results are number n (percentage) of patients for categorical variables and median (q1-q3) for continuous variables.
OR: Odds Ratio and 95% CI: 95% Confidence Intervals were obtained using univariate logistic regression.
Day 1 is the first day of admission in Intensive Care Unit.
NIV: Non Invasive Ventilation; MV: Mechanical Ventilation; SAPSII: Simplified Acute Physiology Score; SOFA: Sepsis-related Organ Failure Assessment.
Table 4: Clinical features of patients the day of ICU admission who needed more than 4 days of non-invasive ventilation who recovered vs. those who failed and finally required mechanical ventilation.
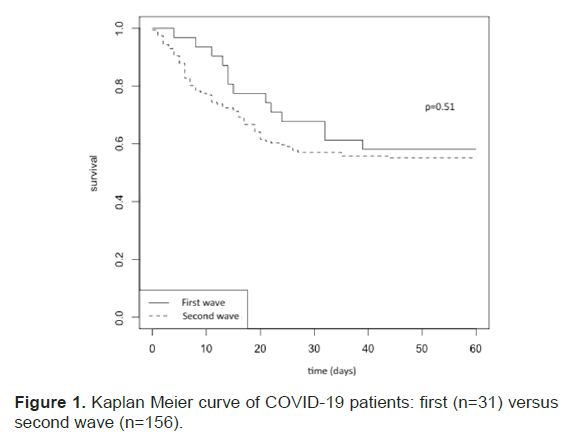
Figure 1: Kaplan Meier curve of COVID-19 patients: first (n=31) versus second wave (n=156).
The study of the delay between dexamethasone and IMV revealed a subgroup of patients characterized by high case fatality rate (89%, n=16/18) with a significant difference (p=0.04) when comparing patients under mechanical ventilation 4 days after dexamethasone onset versus patients under mechanical ventilation less than or equal 4 days after dexamethasone onset (Figure 2).
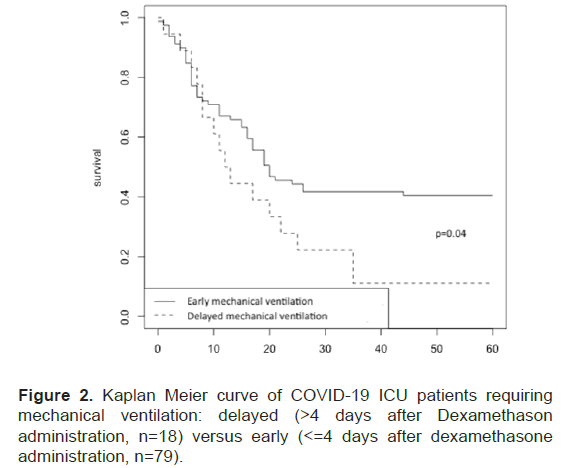
Figure 2: Kaplan Meier curve of COVID-19 ICU patients requiring mechanical ventilation: delayed (>4 days after Dexamethason administration, n=18) versus early (<=4 days after dexamethasone administration, n=79).
We report here a cohort of patients corresponding to the two first waves of the COVID-19 outbreak from the ICU of the University Hospital in Guadeloupe. Despite improvement in terms of ventilatory support and treatment severe COVID-19 pneumonia continued to be grieved with a high mortality rate.
Overall mortality was 44% and was higher in the elderly and those with multiple organ dysfunctions, as previously reported [2]. Unexpectedly, mortality rate were similar in both waves, even after dexamethasone became part of standard of care in the second wave for patients requiring oxygen.
These results should however be interpreted with caution due to several potential biases. First, there could be a “magnifying glass” effect for the second wave patients. In our ICU, admission criteria were tightened and only patients requiring high flow oxygen or immediate IMV were admitted at that time.
SAPS II is known to poorly predict severity of ARDS and that could explain the similarity of patients at baseline between the two waves (p=0.87). Secondly, in our institution, second wave was characterized by >100% COVID-19 patient bed occupancy, at contrary of the first wave. During periods of care system overload, mortality tends to be higher compared to less strained ones [16]. This could also be one of the explanations for similar mortality rates.
Strikingly, in our center, need for IMV were similar in both waves (64% of the patients), despite the more systematic use of steroids in the second wave. Rates of IMV was nevertheless lower than previously described in other centers [2,17].
In univariate analysis, VAP occurrence was associated with higher mortality. Its increased frequency in COVID-19 patients compared to standard ARDS patients has been previously described [18,19]. Consistent with these datas, our incidence of VAP in IMV patients was very high (50%), with no statistical differences between the two periods however the length of IMV was longer during the second wave.
Our multivariate analysis among patients under IMV with respect to survival revealed several factors of interest. As previously described, older age, comorbidities and severity at admission assessed by SOFA score were highly predictive of death. The burden of these factors is well known and has been extensively discussed elsewhere [2,17].
More interestingly, delay between admission to the ICU and IMV onset was predictive of mortality in patients. In the subgroup whose IMV began more than four days after admission to the ICU (n=18, 10% of patients), mortality was very high (n=16, 89%).
Several explanations for this finding are possible. First, like many physicians, we used NIV in COVID-19 patients with hypoxemia. Independent of COVID-19, poor prognosis after failure of NIV has already been described in ARDS patients and with a potentially worsening of lung damage due to self-inflicted lung injury [20,21]. In this pandemic, NIV has been recommended by other authors for COVID-19 pneumonia management 19, but we believe that this should be done with caution with early reappraisal to avoid late IMV.
Secondly, besides this physiological explanation, we believe that such grim outcomes could also reflect the pulmonary fibrotic evolution of COVID-19 pneumonia. This pathological finding has already been described in earlier reports [22,23]. Similarity of mechanical ventilation measurements were observed in this subgroup compared to patients with idiopathic pulmonary fibrosis (data not shown). Larger studies are needed to confirm this result due to its potential impact [24].
We acknowledge several limitations to our study. First, it is monocentric. However, high standardization of COVID-19 care in our unit and results in accordance to larger cohorts advocate for its reliability. Second, the two periods were highly dissimilar in terms of strains on resources. Second wave was characterized by intense clinical activity and overloaded care system.
In this study of 187 critically ill patients with laboratory-confirmed COVID-19 admitted to our ICU, overall, 60-day mortality was 44% with no significant difference between the first and second waves. Mortality increased with the number of comorbidities, delayed mechanical ventilation and the SOFA score.
Our research was approved by our local ethics committee. All patients or families were informed of the data collection.
We give consent for publication.
All datas and materials are available.
No fee was received for this study and we declare no competing interests.
No fundings.
JDP and LC contributed equally to data collection and writings. LF and FVR helped for data collection. FM, KB, MV and MC helped for manuscript writings.
We wanted to acknowledge all doctors, residents, nurses, caregiver in charge of patients during all COVID-19 outbreaks in the intensive care unit of Guadeloupe. We especifically want to thanks our colleagues Dr. Pons, Dr. Piednoir, Dr. Fleury, Dr. Ardisson, Dr. Lawson, Dr. Paulo, Dr. Delamare, Dr. Riaud, Dr. Amouyal and Dr. Elie and all the physicians who came from oversea to help us faced the work overload.
Citation: Pommier JD, et al. A Tale of a Two Waves Epidemic: Characteristics and Mortality Risk Factors for COVID-19 ICU Patients in the French West Indies. J Biol Today's World, 2021, 10(3), 001-006.
Received: 15-Mar-2021 Published: 05-Apr-2021, DOI: 10.35248/2322-3308.21.10.007
Copyright: © 2021 Pommier JD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.