Review Article - (2021) Volume 10, Issue 2
Abiotic stress such as drought, temperature and salinity are the major constraints that limit the worldwide production of maize (Zea mays L.). The development of stress tolerant crop varieties will be beneficial in areas prone to different stresses. Recently, several progresses have been made in identifying genes that are responsible for stress tolerance. Photosynthesis pigments are damaged by drought stress which reduces the light absorption efficiency. NAC proteins are associated with drought tolerance in plants. Indel-572, located 572-base pair upstream of start codon of ZmNAC111, is significantly associated with seedling survival rate. The application of 2(3, 4-Dicholorophenoxy) Triethylamine (DCPTA) improved growth and photosynthetic activity of plants under drought stress. The effect of drought stress was more severe to maize plants compared to heat stress. The major cause of saline soil is the high concentration of NaCl. The plant growth and productivity in saline soil is retarded mainly due to ion toxicity and osmotic stress. Increase in global temperature has shorten crop growing period which can be alleviated by planting varieties requiring more days to mature.
Drought • Temperature • Salinity • Photosynthesis • Ion toxicity • Osmotic stress
Maize (Zea mays L.) is an important staple cereal crop grown for food, feed and biofuel, production all around the globe [1]. It is the third most important crop grown after rice and wheat [2]. To meet the increasing demand for animal and human consumption, studies have suggested that the maize production must double especially in developing nations. The optimum temperature range responsible for higher maize production is 28°C to 32°C and it requires 500 to 800 mm of water to complete life cycle [2].
Environmental conditions play vital role in crop production. The yield and other characteristics of plants are not only determined by their genotypes but are highly influenced by the environmental conditions. Under natural condition, plants undergo different phases to complete their life cycle. In recent years, the climatic parameters such as precipitation, temperature are being more unpredictable and resulted in prolonged drought, change in temperature beyond optimal state. Such changes have challenged crop production. In last two decades the crop productivity has improved, however susceptibility of plants towards abiotic stress possess a new challenge to sustain increase in crop production with changing climatic pattern [3]. Abiotic stress tolerant crops may be essential to sustain crop productivity in future [4]. Plant cells activate signaling pathways that includes plant hormones, transcription regulators, signal transducers in response to various stress. These multiple signal converges to regulate stress inducible genes which in turn produce proteins and enzymes for stress metabolism [5].
Maize production is threatened by increase in moderate to severe droughts, high air temperature, and erratic rainfalls [6]. At present, the major focus of maize research is to improve abiotic stress tolerance characters. However, it’s challenging to identify genetic components responsible for abiotic stress tolerance [3]. Different complex quantitative traits potentially in correlation with other developmental traits are responsible for abiotic stress tolerance. These traits are governed by multiple quantitative trait loci (QTL) with small individual effects on the overall trait expression which makes it more difficult for its identification and modification [1]. The focus of this review is to assess the impact of different abiotic stress in maize production.
Drought is complex and destructive in plant biology to such extend that it is compared with cancer in mammalian biology [7]. The effect of drought varies with timing and intensity of stress on plant’s growth and development [8]. The 2011 drought in southern plains and southwest region of US was an example elucidating the severity of losses caused by drought. Because of this drought and heat waves US agriculture sector suffered loss of more than US $5 billion [9].
Maize is a drought sensitive crop, mainly in critical stage of growth such as seedling stage and is grown in wide range of climatic conditions from semi-arid to temperate regions including drought prone areas of Africa, North and South America, Asia and Europe [2]. Drought stress during vegetative growth especially during V1 to V5 reduces plant growth, increases the vegetative growth period and reduces growth period of reproductive stage [10]. The relative water content and water potential is reduced under stressed condition. This results in increase of leaf temperature because of decrease in transpiration cooling (Figure 1).
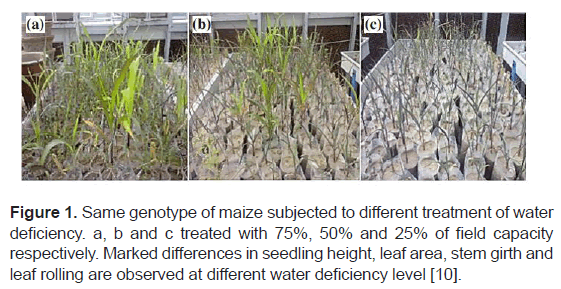
Figure 1: Same genotype of maize subjected to different treatment of water deficiency. a, b and c treated with 75%, 50% and 25% of field capacity respectively. Marked differences in seedling height, leaf area, stem girth and leaf rolling are observed at different water deficiency level [10].
Plants undergo morphological and physiological changes under drought stress condition. This process can be covered under three major categories. They are drought escape, drought avoidance and drought tolerance. The combined impact of these strategies is drought resistance [11]. According to Osmolovskaya et al. drought resistance is ability of plants to maintain favorable water balance and turgidity under water stress condition. Drought escape is a strategy in which plant complete its life cycle before the onset of drought. They show seasonal response [11,12]. Drought avoidance strategy integrates increase water uptake and decrease water loss by plants. Plants develop strategies such as osmotic adjustment, extension of antioxidant capacity, and development of desiccation tolerance in order to develop drought tolerance (Figure 2).
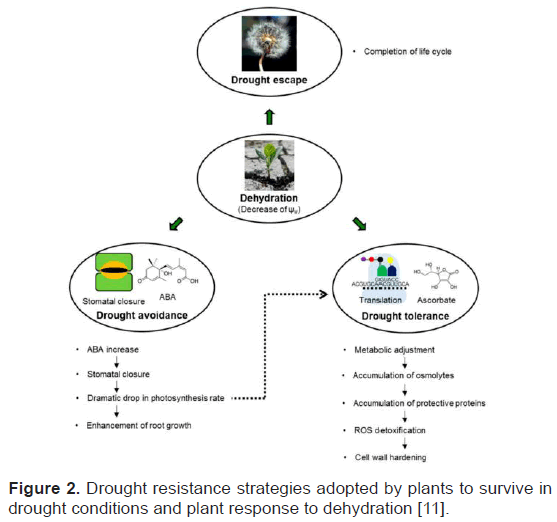
Figure 2: Drought resistance strategies adopted by plants to survive in drought conditions and plant response to dehydration [11].
Different research has been conducted in order to identify drought tolerant varieties. Along with advancement on technology the research focus has changed from morphological characterization to identification of genes responsible for drought tolerance. Photosynthesis, a major metabolic pathway in plants, is sensitive to drought stress and is involved in plant response to drought stress [13]. The photosynthetic pigments are damaged by drought which decreases light absorption efficiency of plants [2]. Though stomata closure is a way forward to ameliorate adverse effects of drought but decrease in stomata opening reduces the amount of CO2 entering in leaves which in turn reduces carbon assimilation reaction and also reduction in transpiration decreases root absorbance.
The plants are found to develop stress tolerance upon external application of plant growth regulators. Xie et al. studied the role of 2-(3, 4-Dicholorophenoxy) Triethylamine (DCPTA) on drought stress tolerance in maize seedlings [2]. The result showed that the application of DCPTA improved growth and photosynthetic activity of plant under drought stress condition. The inbred lines from tropical and sub-tropical regions had higher survival rates as compared to inbred lines from temperate region. It shows higher drought tolerant in the region of maize origin.
NAC protein responses to abiotic stress along with regulation of multiple biological processes in plants [14]. The natural variation in maize drought tolerance was significantly associated with insertion of miniature inverted repeat transposable element (MITE) in promoter of NAC gene (ZmNAC111) [3]. Using genomic wide association study (GWAS), Indel-572, located 572-base pair upstream of start codon of ZmNAC111, was found to be significantly associated with seedling survival rate.
In Nepal, commercial genotypes and local landraces are evaluated in stressed and normal growing conditions. Among nine different lines, Arun- 2 showed better germination and seedling performance when subjected to different level of osmotic potential (0,-5,-10 and -15 bar) in artificially induced drought stress using Polyethylene Glycol (PEG) [15].
Maize plants showed decrease in net photosynthesis (Pn) at leaf temperature above 38°C and the reduction was severe when the temperature increased abruptly rather than gradually [15]. Transpiration rate increased with increase in temperature which implied that decrease in photosynthesis was not because of stomata closure. Increase in temperature greater than 32.5°C decreased the activation state of rubisco which guided to complete inactivation at 45°C [16]. With increase in leaf temperature, the level of 3-phosphoglyceric acid decreased. Rubisco activation acclimatized with increase in leaf temperature and the acclimation process was associated with expression of new activase polypeptide. Crafts-Brander and Salvucci concluded that the primary constraint responsible for decrease in net photosynthesis at temperature greater than 30°C was inactivation of rubisco [16].
Maize is sensitive to chilling injury (below 15°C) and shows less adaptation growing in low temperatures [17]. Miedema found that 36% of the imbibed seeds died when exposed to 4°C for 28 days [18]. Exudation of sugar and amino acid at lower temperature may be associated with dysfunction of cell membranes at lower temperature. Young seedlings died while exposing to 1°C for 6 days and 2.5°C for 8 days. Golgi bodies and inner mitochondrial membrane destructed, endoplasmic reticulum reduced and lipid bodies has accumulated after 3 days of chilling treatment.
Maize leaves are most sensitive to chilling injury. Chilling injury induces premature leaf senescence [17]. The combined exposure of maize leaves to low temperature (10°C) and high light decreases CO2 assimilation and leads to irreversible inhibition of photosynthesis [18].
Janda et al. treated young maize seedlings, grown in hydrophobic condition, with 0.5 mM salicylic acid which protected plants in subsequent application of low temperature stress [19]. According to Janda et al. salicylic acid pretreatment decreased catalase activity which increased activity of antioxidant enzymes such as peroxidases, glutathione reductase leading to increased chilling tolerance in young maize plants [19]. In another research found significant reduction in lipid peroxidation in Glycinebetaine (GB) cells compared to control during chilling [20]. This result implies that increase in chilling tolerance may be caused by reduction of lipid peroxidation of the cell membrane in the presence of GB.
Hussain et al. studied the interactive effect of heat and drought stress on maize hybrids [21]. The combined heat and drought stress significantly reduced plant height, shoot fresh weight, shoot dry weight, leaf area, kernels/ear, 100-kernel weight, and grain yield per plant. Effects of drought stress were more severe as compared to heat stress upon individual application of stress [21].
The chlorophyll content was severely affected by heat stress as compared to drought stress. Relative water content significantly reduced under drought condition while heat stress didn’t reduce relative water content significantly. Intercellular carbon dioxide concentration was increased under drought stress and the increase was significant on both heat and drought stress condition. Transpiration rate showed different results upon different stress condition. It increased under heat stress while reduced under drought stress and further reduced under combined heat and drought stress. Total antioxidant capacity (T-AOC) significantly increased under stress condition as compared to normal growing condition. The soluble sugar, heat shock protein and free protein increased under drought and combined heat and drought stress while the concentration of soluble protein decreased under all individual and combined drought conditions.
Heat stress had no influence in nitrogen concentration in root, leaves and stem as compared to control while drought stress significantly reduced leaf nitrogen concentration. The concentration of phosphorus and potassium was not significantly reduced in individual heat and drought stress as compared to normal. However, under combined heat and drought stress condition, concentration of root nitrogen, stem phosphorus, root, stem and leaf potassium were significantly reduced.
Salinity stress is mostly caused by the high concentration of NaCl which induces abiotic stress in plants in both irrigated and non-irrigated condition. According to a global estimation, 20% of cultivated land and 50% of irrigated land is under salinity stress [22]. Salinity stress retards plant growth and productivity, mainly due to ion toxicity and osmotic stress. Thus induced osmotic stress decrease stomata opening which reduces photosynthetic ability (Figure 3) [23].
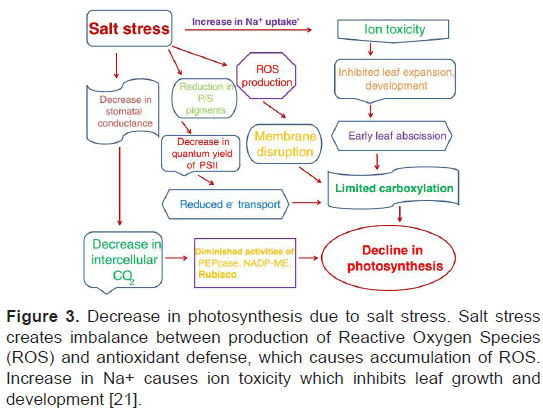
Figure 3: Decrease in photosynthesis due to salt stress. Salt stress creates imbalance between production of Reactive Oxygen Species (ROS) and antioxidant defense, which causes accumulation of ROS. Increase in Na+ causes ion toxicity which inhibits leaf growth and development [21].
Other than limitation in photosynthetic ability, salinity stress causes degradation of enzymatic proteins in photosynthetic apparatus, and chlorophyll degradation [24]. Furthermore, salinity stress causes secondary stresses, in particular oxidative stress, mainly caused by ion toxicity and osmotic stress, which damage plant cell by excessive accumulation of Reactive Oxygen Species (ROS) [23]. Proteins, nucleic acids, lipids and photosynthetic pigments are significantly damaged by ROS. Therefore, antioxidant capacity and photosynthetic capacity are two major elements in salinity stress research programs [25].
Application of exogenous selenium (1 μM) is found to alleviate inhibitory effects caused by salt stress. In an experiment, Jinag studied different concentration of Na2SeO3 (0, 1, 5 and 25 μM) on 15 days old maize plants. This study found that application of 1 μM Se increases net photosynthetic rate, improves antioxidant defense mechanism and reduces chloroplast ultrastructure damage caused by NaCl.
Abiotic stress resistance is mainly accompanied by stress escape, avoidance and tolerance. Plants escape stress by completing their life cycle before the onset of stress. Plants avoid stress, especially heat and drought stress, either by reducing water losses or by increasing water uptake. Plants tolerate stress by maintaining their growth and development along with economical grain yield under stress condition.
The effects of abiotic stress on maize are prevalent form seed germination to maturity. Different genotypes of maize are affected at different level by abiotic stress because of high variability in genetic background. The local landraces and the wild relatives of modern cultivars are found to be resistant to abiotic stress as compared to modern varieties. Thus, the variation can be exploited to develop resistance in commercial maize genotypes.
The combined managerial and biological strategies are the way forward for the development of abiotic stress resistance. Managerial strategies include wise use of water and other resources and development of water saving agronomic practices. Whereas, biological strategies involves manipulation of genetic background of maize for improvement against abiotic stress. Biological strategies comprise conventional breeding, mutation breeding, marker assisted breeding and genomi selection breeding. The development and practical application of different novel breeding techniques will help to reduce problems created by abiotic stress and create food secure condition.
Citation: Bhusal B, et al. A Review on Abiotic Stress Resistance in Maize (Zea mays L.): Effects, Resistance Mechanisms and Management. J Biol Today's World, 2021, 10(2), 001-003.
Received: 28-Jan-2021 Published: 19-Feb-2021, DOI: 10.35248/2322-3308.21.10.006
Copyright: © 2021 Bhusal B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.